Table 1. Catalyst identification and optimization of reaction conditions a .
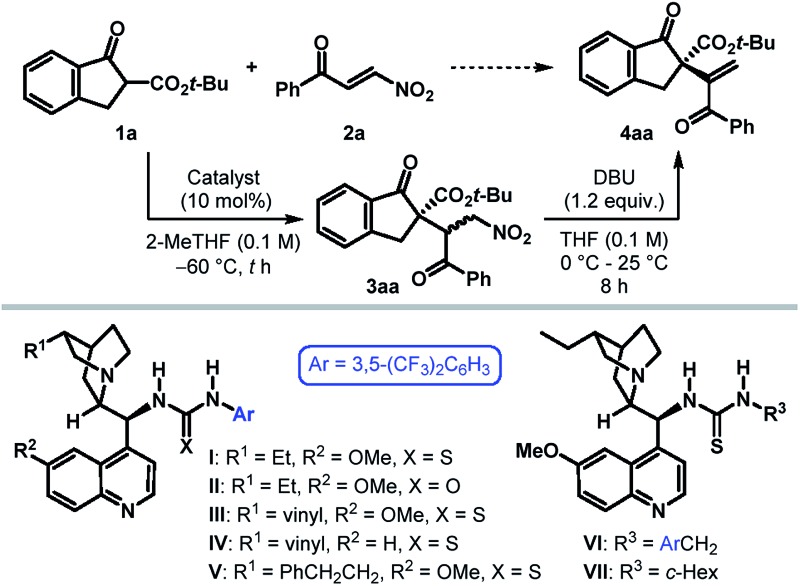
| |||||
| Entry | Catalyst | t [h] | Yield [%] of 3aa b | Yield [%] of 4aa b | er of 4aa c |
| 1 d | None | 6 | 90 | 90 | — |
| 2 | None | 48 | <5 | — | — |
| 3 | I | 48 | 88 | 85 | 94.5 : 5.5 |
| 4 | II | 46 | 95 | 87 | 94 : 6 |
| 5 | III | 48 | >99 | 86 | 92 : 8 |
| 6 | IV | 48 | 90 | 86 | 92 : 8 |
| 7 | V | 48 | 90 | 85 | 93 : 7 |
| 8 | VI | 48 | >99 | 88 | 70 : 30 |
| 9 | VII | 24 | >99 | 88 | 97.5 : 2.5 |
| 10 e , f | VII | 24 | — | 48 | 97.5 : 2.5 |
| 11 e , g | VII | 24 | — | 91 | 97.5 : 2.5 |
aReactions were carried out on a 0.1 mmol scale using 1.0 equiv. of 1a and 1.2 equiv. of 2a.
bYields correspond to the isolated product.
cThe enantiomeric ratio (er) was determined by HPLC analysis.
dReaction at 25 °C.
eUsing 2.0 equiv. of DBU.
fOne-pot reaction in 2-MeTHF. Time required for the 2nd step was 40 h.
gOne-pot reaction with solvent switch.
