Fig. 9.
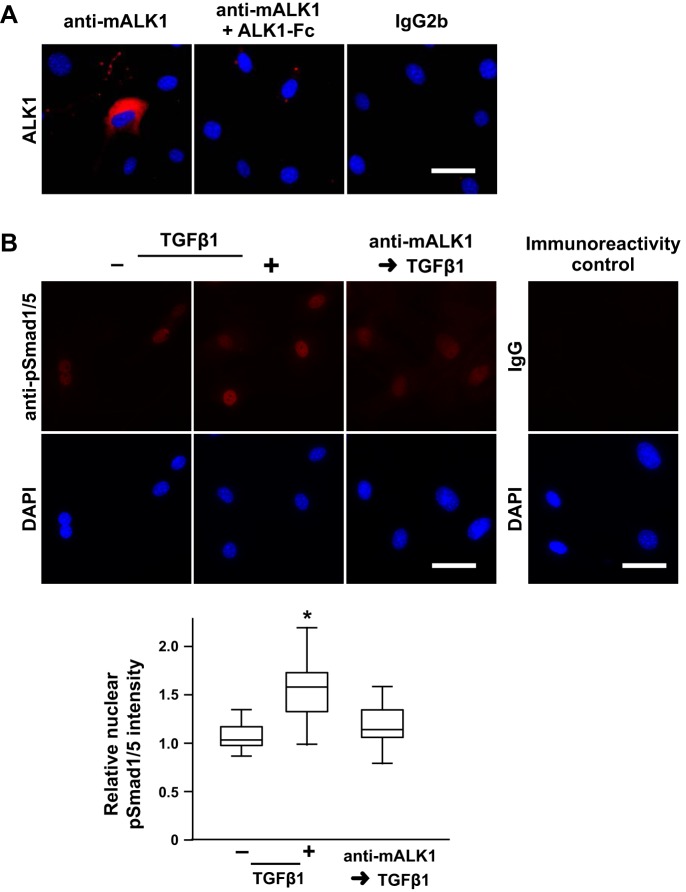
ALK1 regulates TGFβ-stimulated pSmad1/5 nuclear localization in mPASMC. A: ALK1 immunoreactivity is detected on the surface of mPASMC unless the antibody is preadsorbed with solubilized extracellular ALK1 domain (mALK1-Fc). Live cells were reacted with an anti-mALK1 antibody, an IgG2b monoclonal rat antibody that was generated against an extracellular fragment of mouse ALK1, without and with previous exposure to mALK1-Fc, or rat IgG2b, washed, fixed, and then reacted with a fluorescently labeled secondary antibody and DAPI. Subsequently, epifluorescence microscopy was performed. Typical images of two or three independent studies are shown. B: pretreatment with an anti-mALK1 antibody inhibits TGFβ-mediated BMP R-Smad phosphorylation. Cells were treated with 0 or 15 µg/ml anti-mALK1 and then incubated with 0 or 2.5 ng/ml TGFβ1 for 1 h. Subsequently, the cells were fixed and pSmad1/5 immunoreactivity, and DAPI reactivity was assessed. In additional immunoreactivity control studies, the cells were reacted with an isotype control instead of the anti-pSmad1/5 antibody. The nuclear immunoreactivity intensity signal in a region of interest identified by the DAPI reactivity was quantified and normalized with the mean level detected in the control cells. n = 45 per group; *P < 0.05. Scale bars = 50 µm.