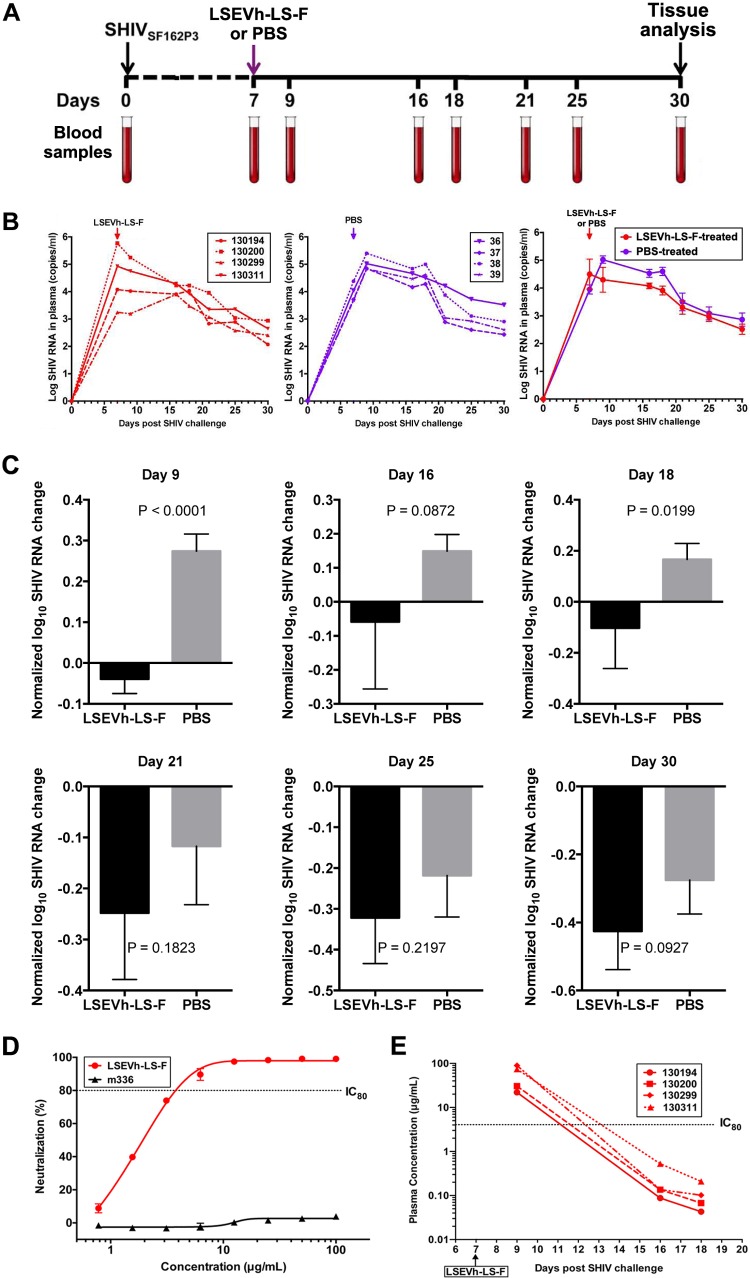
FIG 6.
Effect of LSEVh-LS-F treatment on plasma SHIV loads. (A) Experimental treatment and analysis scheme. (B) Eight rhesus macaques were challenged intravenously with SHIV-1SF162P3 and treated on day 7 with either a single infusion of LSEVh-LS-F (n = 4) or PBS (n = 4). Plasma SHIV loads from individual LSEVh-LS-F-treated macaques (left panel) and individual PBS-treated macaques (middle panel) and the average for the four macaques in the LSEVh-LS-F-treated and PBS-treated groups (right panel) are shown. (C) Analysis with normalized values of the data shown in panel B was performed to decrease the effect of individual macaque variation. Evaluation of the difference in plasma SHIV loads between the treatment and control groups was analyzed by the unpaired t test for different days after treatment. The decrease of virus RNA at day 9 for LSEVh-LS-F-treated compared to PBS-treated macaques is highly statistically significant (P = 0.0001). (D) Dose-response in vitro neutralization of SHIVSF162P3 by LSEVh-LS-F assayed in TZM-bl cells. An irrelevant monoclonal antibody, m336, was used as the negative control. (E) Concentration of LSEVh-LS-F after a single infusion of LSEVh-LS-F as a function of time for the individual treated macaques. The limit of detection is about 0.1 μg/ml, and after day 18, the LSEVh-LS-F concentration can be assumed to be undetectable.