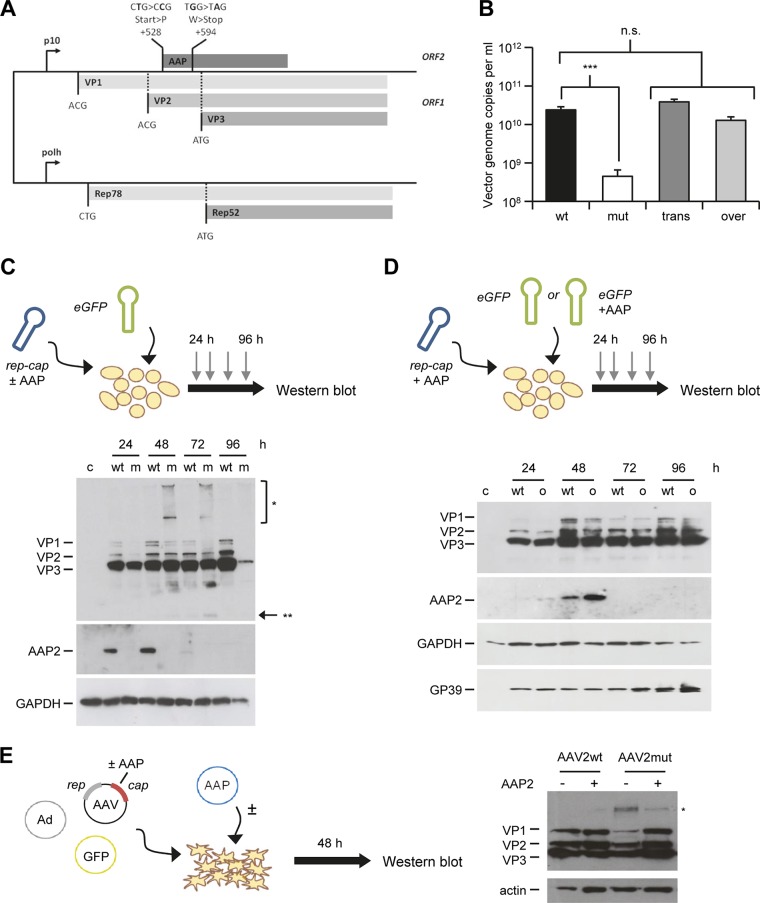
FIG 2.
Role of AAP in AAV2 assembly in insect cells. (A) Generation of AAP2 knockout recombinant baculovirus. AAP2 was knocked out through mutation of the AAP2 start codon and insertion of a stop codon, impairing the AAP2 ORF but retaining the cap ORF, similarly to the case for AAP knockout AAV2 helper plasmid (Fig. 1A). BEV-Rep2Cap2-AAP2mut was generated as described in Materials and Methods using the Bac-to-Bac technology. (B) AAP2 trans-complementation and overexpression in the dual-baculovirus production system (n = 3). Sf9 cells were coinfected with BEV-AAV and BEV-RepCap at an MOI of 0.05 per baculovirus. Conditions “wt” and “mut” correspond to coinfection with BEV-eGFP-Puro and BEV-Rep2Cap2 or BEV-Rep2Cap2-AAPmut, respectively. Condition “trans” corresponds to infection with BEV-eGFP-Puro-p10AAP2 expressing AAP2 in trans and BEV-Rep2Cap2-AAP2mut. For the “over” condition, Sf9 cells were infected with BEV-Rep2Cap2 and BEV-eGFP-Puro-p10AAP2. Vector genomes in crude cell lysates were quantified at harvest by free-ITR qPCR (see Materials and Methods). Statistical analysis was by a one-way ANOVA with Bonferroni's multiple-comparison test. n.s., not significant; ***, P < 0.001. (C) Time course of AAP2 and VP expression during AAV2 vector production in insect cells. Sf9 cells were coinfected with BEV-eGFP and BEV-Rep2Cap2 (wt) or BEV-Rep2Cap2-AAPmut (m) at an MOI of 0.05 per baculovirus. Western blotting was performed from 10 μg of total proteins extracted from cell pellets of uninfected (c, control) or infected Sf9 cells recovered every 24 h postinfection. AAP2 or VP proteins were detected using the anti-AAP2 or polyclonal anti-VP antibodies, respectively. The asterisks indicate high-molecular-weight (*) or low-molecular-weight (**) signals from the anti-VP antibody; the latter may represent degraded VP proteins. (D) Time course of AAP and VP expression during AAV2 vector production with AAP2 overexpression. Sf9 cells were infected with BEV-Rep2Cap2 and BEV-eGFP-Puro (wt) or BEV-eGFP-Puro-p10AAP2 (o, overexpression) at an MOI of 0.05 per baculovirus. Western blotting was performed, and AAP2 and VP proteins were detected, as for panel C. SDS-polyacrylamide gel loading and BEV infection were verified with anti-GAPDH and anti-GP39 antibodies, respectively. (E) Detection of B1 antibody-reactive, high-molecular-weight bands in mammalian cells expressing AAV2 VP proteins in the absence of AAP2. Shown is a representative Western blot analysis of lysates from HEK293T cells transfected and treated as for Fig. 4B, left panel. The blot was overexposed to better illustrate the high-molecular-weight signal (asterisk) that is most pronounced in the AAV2mut sample in the absence of AAP2 (third lane).