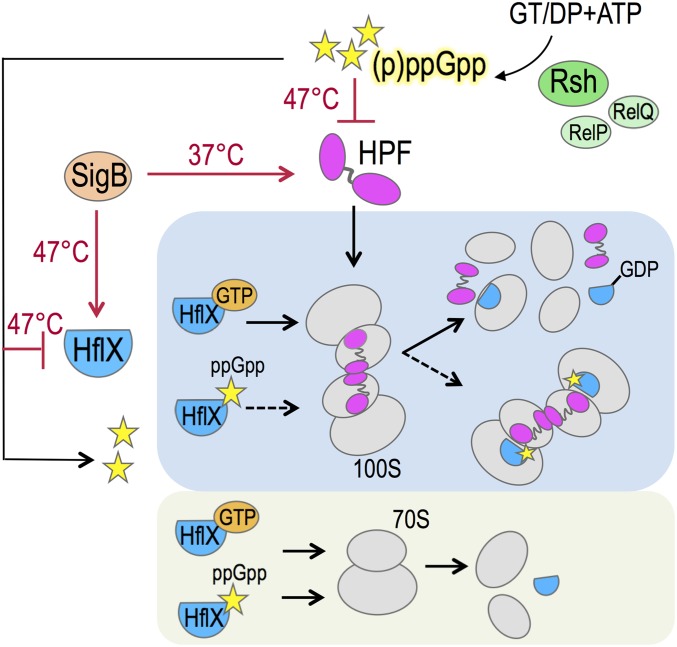
Fig. 1.
A model summarizing the coregulation and opposing roles of HPF and HflX. The stringent response alarmone (p)ppGpp in S. aureus is synthesized from the substrates GT(D)P and ATP primarily by the Rsh (RelA/SpoT homolog) enzyme and, to a lesser extent, by two alternative synthetases, RelP and RelQ (55). The N-terminal domain of HPF binds to the decoding center of the 30S subunit and inhibits translation, whereas the C-terminal domain (CTD) tethers the two 70S monomers via direct interaction of the HPF-CTD dimer to form the 100S complex (19). The production of (p)ppGpp strongly inhibits the synthesis of hpf and hflX under heat stress. ppGpp also binds to HflX. HflX⋅ppGpp is unable to split the 100S complex but is sufficient for 70S dissociation. HflX binds to the peptidyltransferase center in the 50S subunit and stimulates subunit dissociation by disrupting intersubunit bridges (46). The effective stoichiometry of HflX⋅GTP-100S remains to be determined. GTP hydrolysis presumably promotes the release of HPF and HflX simultaneously with 100S breakdown, possibly by way of a 70S intermediate. The general stress response sigma-factor B (SigB) activates the expression of hpf at 37 °C and moderately up-regulates the HflX level at 47 °C. Red arrows indicate a positive regulatory role, bar-headed lines denote repression, and a dashed arrow indicates a loss of action.