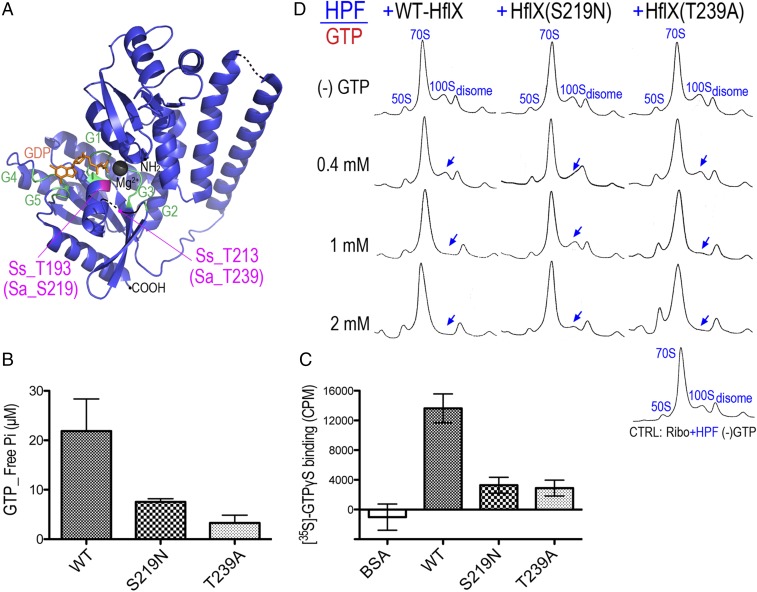
Fig. 3.
GTP hydrolysis is required for the HflX-mediated disassembly of the 100S ribosome. (A) Crystal structure of archaeon S. solfataricus HflX in complex with GDP (orange) (Protein Data Bank 3KXI). Residues critical for GTP binding/hydrolysis are colored in magenta, and equivalent positions in the S. aureus HflX are denoted as Sa_S219 and Sa_T239. Magnesium is in gray. G1–G5 domains are colored in green. Dashed lines denote the unstructured regions in the crystal. Overall sequence homology (34%) between SsHflX and SaHflX is shown in Fig. S1. (B) Malachite green GTPase assay showing the reduction of GTPase activity in the S219N and T239A mutants. Each reaction contains 4 μM WT HflX or its mutants and 0.4 mM GTP substrate for a total of 200 μL. The amount of free inorganic phosphate (Pi) released upon GTP hydrolysis was calculated from a Pi standard curve. Error bars are SEs from four independent experiments using three different batches of purified proteins. (C) The S219N and T239A mutants are impaired in GTP binding. Filter binding assays of HflX variants (5 μM) mixed with 0.2 μM of nonhydrolyzable [35S]-GTPγS. The amount of radiolabeled GTP [expressed in counts per million (cpm)] bound to the nitrocellulose membrane-adhered HflX was measured on a liquid scintillation counter. The same amount of non-GTP binder BSA was used in place of HflX to serve as a negative control. The y axis represents the values after background (reaction without any protein) subtraction. Error bars are SEs from three independent experiments. (D) The S219N and T239A mutant proteins do not dissociate the 100S ribosome as efficiently as wild-type (WT) HflX. Shown are 5–25% sucrose gradient density profiles of the in vitro 70S dimerization reactions treated with either WT HflX or its catalytic mutants in the presence of a range of GTP. The entire reaction includes 0.2 μM ribosomes and HPF, 2 μM HflX, and 0–2 mM GTP. Blue arrows mark the diminishing 100S peaks.