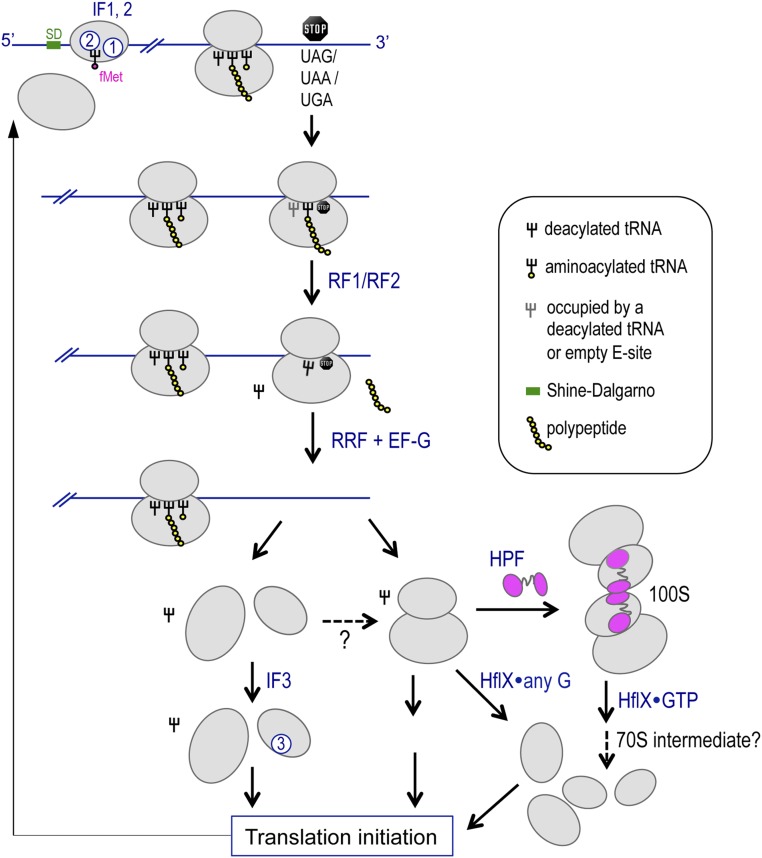
Fig. S7.
A simplified model depicting possible relationships of RRF, IF3, and HflX during ribosome recycling. The initiation of bacterial protein synthesis involves the recruitment of initiation factor 2 (IF2), IF3, mRNA, and the initiator fMet-tRNAfMet to the 30S subunit. IF2 and IF3 eventually dissociate, and the 50S subunit binds to form the initiation complex with the initiator tRNA position at the P site. Subsequent elongation steps are facilitated by the actions of EF-Tu, GTP, and aminoacylated tRNAs with the aid of EF-G to promote translocation. When a termination codon enters the decoding center, it is recognized by the cognate release factor 1 (RF1) or RF2. RF1 and RF2 catalyze the hydrolysis of the peptidyl–tRNA bond, thus liberating the newly synthesized protein. RF3 promotes the dissociation of RF1 and RF2 from the ribosome. During the recycling phase, ribosome recycling factor (RRF) and GTPase EF-G concertedly stimulate the dissociation of the 70S ribosome from the mRNA, followed by tRNA release and in most cases dissociation of the 30S and 50S subunits (35). The binding of IF3 to the 30S subunit prevents 30S–50S rejoining. Without IF3, the subunits may reassociate to form the silent 70S complex. HflX disassembles the 70S complexes released from mRNA and from subunit rejoining in the presence of guanosine binding. HPF dimerizes the 70S complexes into translationally inactive 100S ribosomes. Under specific conditions, GTP-bound HflX dissociates the 100S ribosome into subunits with the possibility of a 70S intermediate during disassembly and with the dislodging of HPF and HflX upon GTP hydrolysis. The free subunits can be recycled for new rounds of translation.