FIGURE 2.
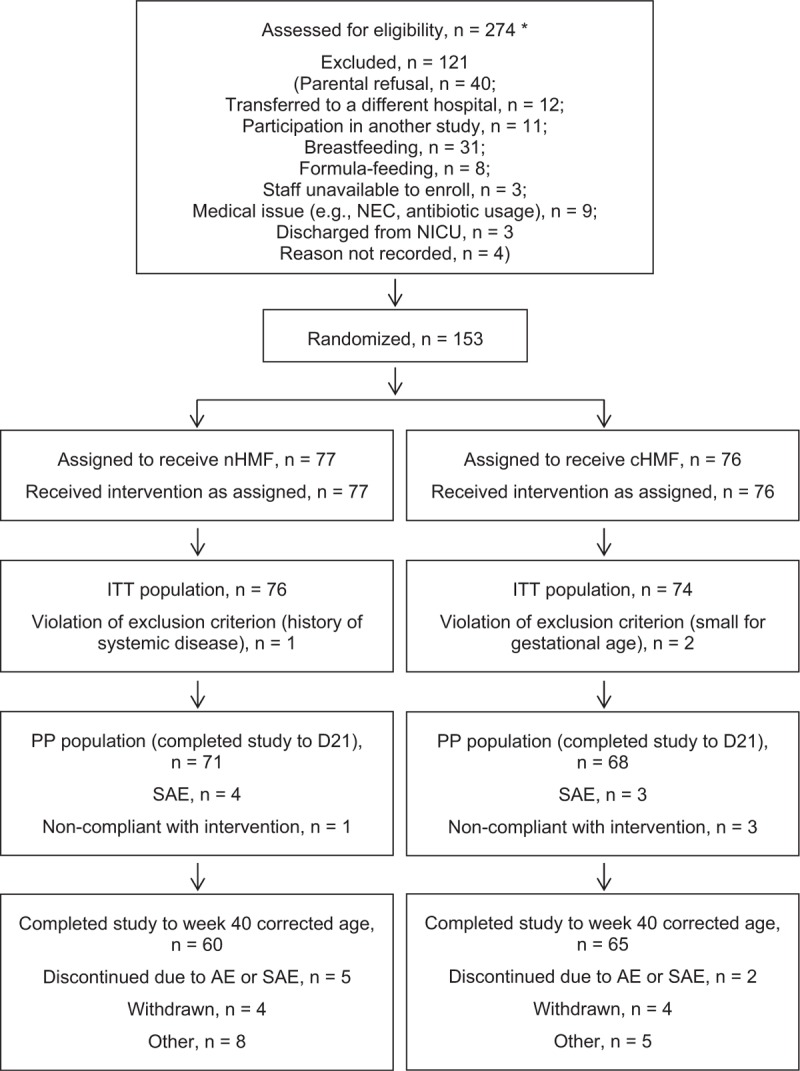
Flow of study participants. AE = adverse event; cHMF = control human milk fortifier; D21 = study day 21; ITT = intent-to-treat; NEC = necrotizing enterocolitis; nHMF = new human milk fortifier; NICU = neonatal intensive care unit; PP = per-protocol; SAE = serious adverse event. ∗Although screening procedures were standardized across sites, some variability in prescreening procedures did occur. Based on the typical clinical characteristics of infants who were admitted to each NICU during the study interval, the total number of infants who would have been theoretically considered eligible for the study was higher than the number shown here.
