Abstract
Th17-dependent autoimmune responses can develop after heart or lung transplantation, and are associated with fibro-obliterative forms of chronic rejection. However, the specific self-antigens involved are typically different from those associated with autoimmune disease. To investigate the basis of these responses, we questioned whether removal of Tregs or blockade of function reveals a similar auto-antigen bias. We found that Th17 cells specific for collagen type V (Col V), kα-1-tubulin, and vimentin were present in healthy, adult PBMC, cord blood, and fetal thymus. Using synthetic peptides and recombinant fragments of the Col V triple helical region (α1V), we compared Th17 cells from healthy donors with Th17 cells from Col V-reactive heart and lung patients. While the latter responded well to α1(V) fragments and peptides in a DR–restricted fashion, Th17 cells from healthy individuals responded in a DR-restricted fashion to fragments, but not to peptides. Col V, kα-1-tubulin, and vimentin are preferred targets of a highly conserved, hitherto unknown, pre-existing Th17 response that is MHCII-restricted. These data suggest that autoimmunity after heart and lung transplantation may result from dysregulation of an intrinsic mechanism controlling airway and vascular homeostasis.
Introduction
Organ transplantation is the only definitive treatment for many forms of end-stage cardiac and pulmonary disease (1, 2). While advances in the transplantation field have curbed acute rejection through new immunosuppressive drugs and better control of infection and ischemia-reperfusion injury, chronic allograft rejection is still a major obstacle. Successful organ transplantation appears to require a balanced function of effector and regulatory T cells to prevent the emergence of Th17 based fibrosis and fibro-obliterative processes in the allograft (3). Th17 cells have been strongly associated with autoimmune disease, including lupus (4), rheumatoid arthritis (5, 6), psoriasis (7, 8) and multiple sclerosis (9, 10). In addition, Th17 cells have been found to play a key role in the chronic rejection of lung (11, 12), and heart transplants (13, 14).
We have previously reported cellular immune responses to the self-antigen Collagen type V (ColV) in lung and heart transplantation as well as in conditions pre-disposing patients to end-stage organ failure, such as idiopathic pulmonary fibrosis (11, 15) or coronary artery disease (CAD) (12) pathologies. These responses correlated with a greater probability of primary allograft dysfunction (15–17) and chronic rejection of the graft (13). Furthermore, we reported that the cellular immune response to ColV in these patients was Th17 mediated, as the ColV response depended on IL-17, with variable dependence on IFNγ (11–13). Interestingly, TNFα, IL-1β and P2X7R function, both on the Th17 cells and on monocyte-antigen presenting cells (APCs), were also required for the response to ColV in transplant recipients (13).
Besides ColV, the other well characterized self antigen evoking responses in chronic rejection of lung allografts is kα-1-tubulin (18–20). It has been reported that both T and B cell reactivity to this antigen predicts bronchiolitis obliterans in both mouse and human lung transplantation (19). In addition, vimentin, a type III intermediate filament component of mesenchymal cells, has been associated with chronic rejection of cardiac allografts in humans and mice (21, 22).
Recently, a Treg expressing the 3’5’ ecto-nucleotidase, CD39, has emerged as a suppressor of Th17 cells in numerous pathologies (23–26). Expressed on approximately fifty percent of human Tregs, CD39 can suppress both Th1 and Th17 responses (23, 27, 28). Furthermore, CD39 depleted (CD39−) Tregs failed to suppress Th17 responses, implicating a critical role for CD39 in Treg control of autoimmune Th17 cells (27, 28). CD39+ Tregs can rapidly lower levels of extracellular ATP, decreasing P2X7R signaling and increasing the immuno-suppressive purine, adenosine (29–31). This can lead to less IL1β production from monocytes and macrophages and reduced Th17 mediated immune responses (32) (3). In normal individuals, Tregs can modulate auto-immune effector T cell function through suppressive cytokines IL-10, IL-35 and TGFβ (27, 33, 34). This system of Treg-Th17 balance may be deficient in individuals who are undergoing chronic rejection of heart or lung allografts as has been reported in kidney allograft models (35).
Two major questions regarding Th17 mediated auto-immune pathologies remain, the first of these is why is ColV, vimentin or kα-1-tubulin and not the self antigens commonly associated with auto-immune disease the focus of transplant-induced auto-immunity? The second question is whether the pathogenic Th17 cell is induced in the periphery or whether it is a pre-existing-thymically differentiated, Th17 cell (Th17) that has escaped regulation? In this report, we will address the first question—testing the hypothesis that pre-existing Th17 and Treg cells, specific for ColV, vimentin and kα-1-tubulin, are present in normal, healthy individuals.
Methods
Human Subjects
Peripheral blood mononuclear cells (PBMCs) were obtained from 12 donors, both male and female, and ranging in age from 22–60 years old. Blood was collected and PBMCs were processed as previously described (36). Human fetal spleen and thymus (16–17 weeks gestation) specimens from elective and/or medically indicated pregnancy terminations were obtained from the Human Fetal Tissue Repository (HFTR) at Albert Einstein College of Medicine of Yeshiva University (Bronx, NY).
Cell Separations
For isolating CD39− or CD25− T cell populations, PBMCs were first incubated with CD14 (Miltenyi Biotec, San Diego CA; cat# 120-001-146) microbeads to remove monocytes that are observed to have high expression of CD39 or may express CD25. Following monocyte separation, cells were incubated with either pan T-cell (Miltenyi, 120-008-788) microbeads and CD39 microbeads, or pan T-cell (Miltenyi, 120-008-788) microbeads and CD25 microbeads and separated using an autoMACS (Miltenyi) cell separator as per the manufacturer’s protocol. Autologous monocytes were added back to CD39 or CD25 depleted T cell fractions in the same frequency as observed in the whole PBMC sample and used in tvDTH or ICCS assay systems.
For mouse studies, spleen and bone marrow harvested from 10-16 naïve CBA mice from various vendors were used as a source of T cells and monocytes, respectively. Single cell suspensions of mouse splenocytes were attained as previously described (12). For bone marrow harvesting, the tibia and femur of the mice were cut and marrow was flushed out with PBS supplemented with 2% FBS and 1mM EDTA. For mouse monocyte separation experiments from the bone marrow, a negative selection monocyte separation kit from StemCell Technologies (Vancouver, BC cat# 19761) was used in accordance with the manufactures protocol. CD4+ T cells were obtained from mouse splenocytes, using a pan T cell isolation kit (Miltenyi, #130-095-130) according to the manufacturer’s instructions. CD25+ T cells were then depleted from isolated CD4 T cell populations by positive CD25 selection (Miltenyi, # 130-091-072).
Transvivo Delayed-Type Hypersensitivity Assay
The Trans-vivo delayed type hypersensitivity (tvDTH) assay was performed as described previously (11, 37). Inactivated Tetanus Toxoid was purchased from Sanofi-Aventis Pasteur and is used as a positive control antigen for all experiments. Human Col V [5 ug] was a gift from Dr. David Brand (University of Tennessee, Memphis), and was prepared from placenta as described elsewhere (38). Human Col I was purchased from BD Pharmingen. Collagen XI [5ug] was purchased from Chondrex Inc. Vimentin [5ug] was purchased from R&D Systems, and kα-1-tubulin [5ug] was a generous gift from Dr. Mohanakumar at Washington University in St Louis. Prostate Acid Phosphatase [1ug] was a gift from the lab of Dr. Doug McNeel at the University of Wisconsin. Myelin Basic Protein ([10ug], MBP) was purchased from Enzo Life Sciences (Farmingdale, NY). HSP65 [10ug] was purchased from Stressgen Biotechnologies (Victoria, BC, Canada) HMGB-1 [15ug] was purchased from Sigma Chemical (St. Louis MO). Neutralizing antibodies to TGFβ ([25ug], R&D Systems, AB-100-NA), IL-35 ([1ug], R&D Systems, MAB 1570), CTLA-4 ( [1ug], Antibody Solutions, AS32-P), IL-10 ([1ug], R&D Systems, AB-217-NA), IL-17 ([5ug], eBioscience, 16-7178-85), IL-1β ([5ug], eBioscience, 16-7018-85), IFNγ ([5ug], eBioscience, 16-7318-85), TNFα ([5ug], eBioscience, 16-7348-85) or IL-22 ([5ug], R&D Systems, MAB7822) were used along with appropriate IgG [25-5ug] isotype control antibody.
Intracellular Cytokine Staining (ICCS)
PBMCs or MACS separated CD39− T cells and autologous monocytes were cultured in DMEM/5% FBS in the presence of the indicated agonist at 37°C. After overnight incubation, cells were stimulated with PMA (10ng/ml) and Ionomycin (1ug/ml) for 5 hours; the last 3.5 hours of stimulation in the presence of Brefeldin A. Following stimulation, cells were treated with Fc blocker and subsequently stained for surface markers CD3 [BD Biosciences, Franklin Lakes NJ # 561805] and CD4 [BD Biosciences, Franklin Lakes NJ # 561839]. Next, cells were permeabilized and stained for intracellular cytokines (IL-17 [BD Biosciences, Franklin Lakes NJ # 562933], IL-22 [Biolegend, San Diego, CA # 366704] , TNFα [BD Biosciences, Franklin Lakes NJ # 557647] and IFNγ [BD Biosciences, Franklin Lakes NJ # 554702] or transcription factors (RORγt [R&D Systems, Minneapolis MN, #IC6006P], PLZF-1 [BD Biosciences, Franklin Lakes NJ #564850] using the Cytofix/Cytoperm kit (BD Biosciences, Franklin Lakes NJ). Cells were fixed in 2% paraformaldehyde (PFA) and acquired on a LSR II flow cytometer (BD Biosciences, Franklin Lakes NJ). Percentages of CD4 positive, cytokine-producing cells were determined using Flowjo analysis software (Treestar, St. Louis, MO).
Statistical Analysis
Multiple replicates were averaged across each assay and data were combined and analyzed by ANOVA, Mann-Whitney U or paired T test performed with Prism 5.0 (GraphPad Prism software, La Jolla, CA).
Animal Usage and Study Approval
Human subject informed consent was obtained using IRB- approved, informed consent procedures at the University of Wisconsin Hospital and Clinics. For studies using fetal thymus and spleen, all work was done under approval from the UW-Madison IRB and that of Albert Einstein College of Medicine, in accordance with federal guidelines. All samples using mice or rhesus macaques (n=6) blood were performed with animal health and safety guidelines under strict accordance with ACUC and ALAC guidelines.
Results
Col V but not Col I responses are revealed by CD39 or CD25 depletion of T lymphocytes in normal, healthy individuals and mice
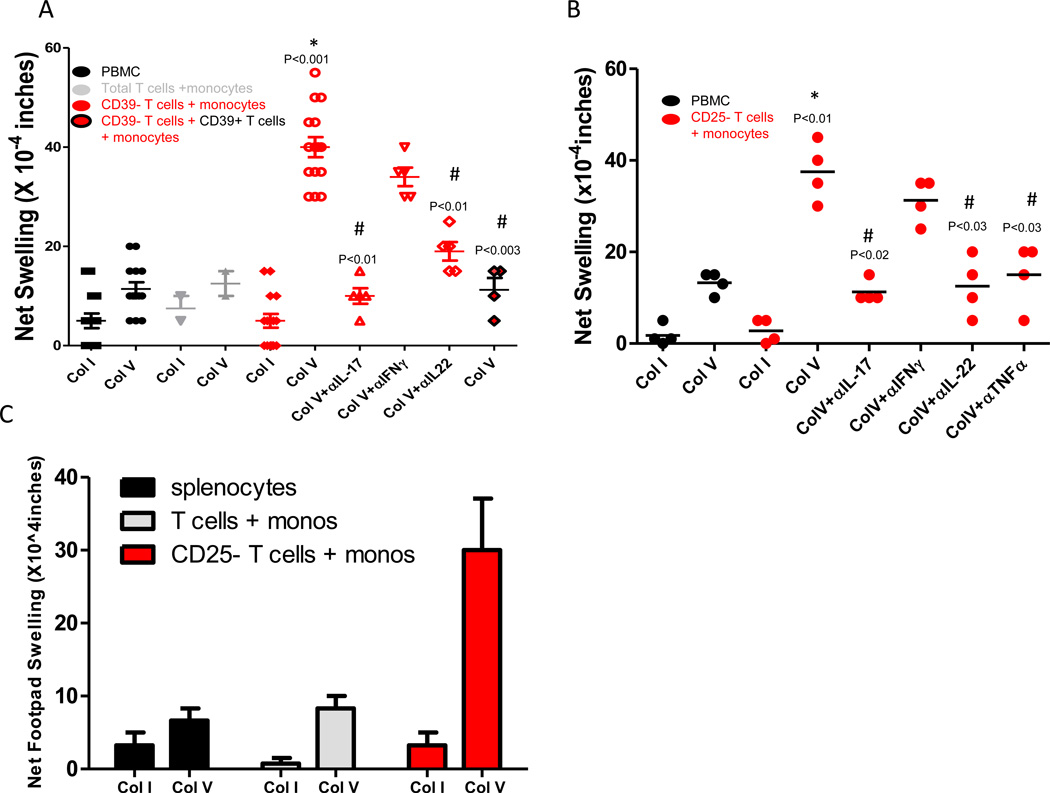
To determine whether Col V specific cellular immune responses can be found in normal, healthy individuals after removal of Tregs, whole PBMCs, total isolated T cells plus monocytes or CD39− T cells plus monocyte mixtures were stimulated with Col I or ColV (Figure 1A). After footpad injection in CB17.SCID mice, swelling responses were measured 24 hours later. In whole PBMCs, and in freshly isolated T cell plus monocyte mixtures, there was no significant difference (p=0.14) between Col I and ColV treatment groups, although a trend toward a higher response to ColV vs Col I was observed. In groups of CD39− T cells plus monocytes, we observed no increase in the cellular immune response to Col I, but ColV stimulation caused a significant increase (p<0.001) in the swelling response. Neutralizing antibodies to Th17 associated cytokines, including IL-17 (p<0.01) and IL-22 (p<0.02) caused a significant reduction in the cellular immune response to ColV. The add back of CD39+ T cells to groups of CD39− T cells plus monocytes restored the low Col V response seen in normal, healthy individuals. Neutralizing antibodies to IFNγ showed a trend toward inhibition of the Col V response in CD39− T cell plus monocyte groups. When we examined whether the removal of CD25+ T cells could reveal a similar ColV specific Th17 response in normal, healthy individuals (Figure 1B), we found that depletion of CD25+ T cells, when combined with autologous monocytes, revealed a ColV specific cellular immune response that differed significantly (p<0.01) from the response to ColV in unseparated PBMCs. CD25+ T cell depletion had no effect on the tvDTH response to Col I. The ColV specific effector response was IL-17 (p<0.02), TNFα (p<0.03) and IL-22 (p<0.03) dependent, as indicated by neutralizing antibodies. Neutralizing antibodies to IFNγ showed a trend toward inhibition of the ColV response. To rule out the possibility that a pre-existing Th17 memory response to ColV is unique to humans, we investigated whether Treg removal could reveal ColV specific cellular immune responses in mice. Single cell suspensions of monocytes isolated from bone marrow were mixed with unfractionated or CD25 depleted T cells isolated from spleens of 6 to 8 CBA mice. Figure 1C illustrates that in whole splenocytes, or total T cells plus monocytes, there was no appreciable increase in the ColV over the Col I response. However, in mixtures of CD25- T cells plus monocytes, there was a 4-fold increase in the ColV response over that observed in whole splenocytes or total T cells plus monocytes, with no increase in response to Col I.
Figure 1. Col V but not Col I responses can be uncovered through depletion of CD39+ or CD25+ T cells in humans and mice.
(A) Whole PBMCs (black), MACS separated monocytes plus T cell subsets (grey) or CD39− depleted T cells and autologous monocytes (red) were tested for footpad swelling responses to Col V and Col I using the tvDTH assay. To test for cytokine-dependency, some footpads were injected with cells plus neutralizing antibody. In some experiments, CD39+ T cells were added back to the footpad-antigen mixture (red and black symbols). P values are shown for comparison with the Col I response or Col V response observed in PBMC group. Data from 2–14 individuals are shown. (B) Whole PBMC (black) or CD25 depleted T cells plus autologous monocytes (red) were tested for footpad swelling responses to Col V and Col I using the tvDTH assay. Data are from 4 individuals. (C) Separations of monocytes, whole T cells and CD25− T cell populations was performed from CBA mouse spleens similar to that described in Methods. Following separations, groups of isolated total T cells plus autologous monocytes and CD25− T cells plus autologous monocytes along with whole splenocyte samples were stimulated with Col I or Col V and used in the tvDTH assay. Results are from 8 pooled mice. Significant differences between groups were determined by Mann Whitney U tests (A, B) or ANOVA (C).
ColV specific IL-17 production in CD4+ T cells from healthy individuals
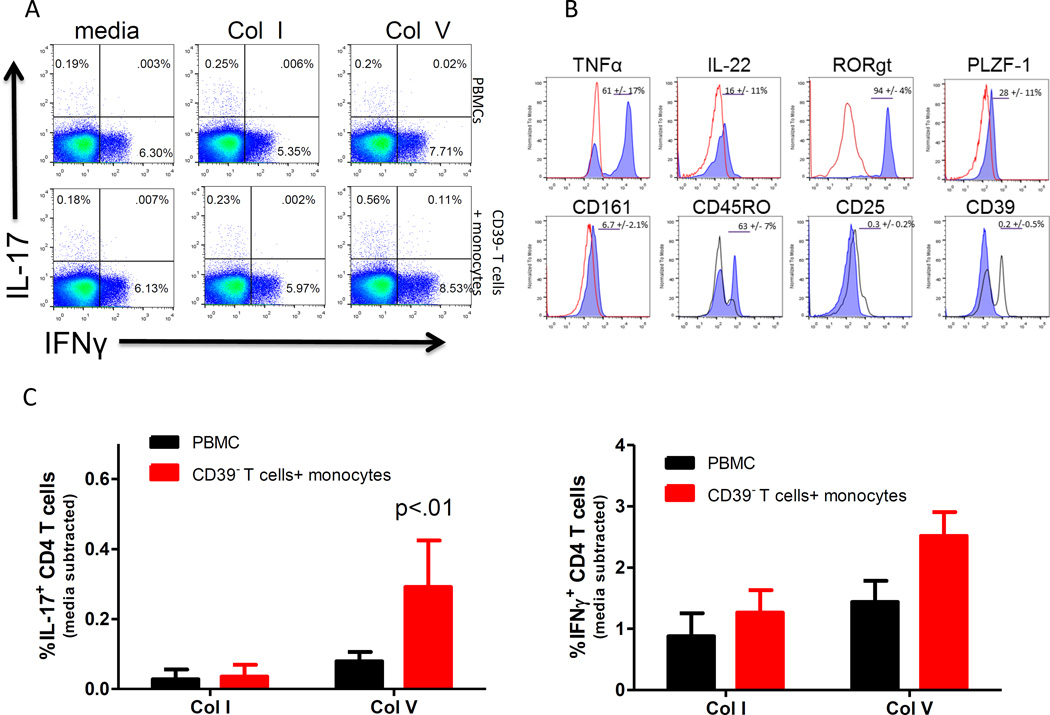
To identify the cells responsible for the pre-existing ColV response, we stimulated overnight cultures of whole PBMC, or CD39− T cells plus monocytes with Col I or ColV followed by PMA/Ionomyocin treatment. Figure 2A shows representative flow plots of intracellular IL-17+ and IFNγ+ cells as a percentage of CD4+ T cells in unseparated PBMCs (top) or CD39− T cells plus monocytes (bottom). Panels B and C show quantified flow cytometry data from 6–9 normal, healthy individuals. Data is expressed as frequency of IL-17 or IFNγ producing CD4 T cells minus media control. While there was no increase in the frequency of IL-17 producing CD4 T cells after ColV stimulation of whole PBMCs, there was a significant increase (p<0.04; Figure 2B) in the corrected frequency (0.2–0.5%) of ColV reactive CD4 T cells in the CD39− T cells plus monocyte culture as compared with Col I or media control cultures. While not significant (p=0.09), there was also a trend toward an increase in IFNγ+ CD4 T cells after ColV stimulation in groups of CD39− T cells plus monocytes, closely paralleling the tvDTH inhibition trend with anti-IFNγ neutralization. We further characterized the IL17+ T cell subset and found that between 40–70% of the Th17 cells were also positive for TNFα, and 15–30% were positive for IL-22 (Figure 2D). The IL-17 responders were negative for TCRγδ (data not shown). Furthermore, the IL-17 responding population was low in CD161, but positive for RORγt, PLZF-1 and CD45RO.
Figure 2.
Col V specific IL-17 production from normal, healthy individuals is revealed when CD39+ T cells are removed. (A) Representative flow plots of IL-17 and IFNγ production from overnight cultures of whole PBMCs (top) or CD39− T cells plus autologous monocytes (bottom) stimulated with Col I or Col V from a normal, healthy individual. Inset numbers represent percentage in each quadrant. (B and C) Quantified flow cytometry data from panel A indicating frequencies of IL-17 or IFNγ producing CD4 T cells from 7–10 normal, healthy individuals. P value represents significance vs PBMC-Col V stimulated control. (D) Representative histograms of TNFα, IL-22, PLZF-1, RORγt, CD45RO, CD161, CD39 and CD25 (blue) expression gated from total IL-17+ populations of Col V stimulated cells from the CD39− Tcells plus monocyte groups. Red line represents isotype control (TNFα, IL-22, PLZF-1, RORγt, CD161), black line represents staining from CD3+/CD4+ T cells that are both IL-17 and IFNγ negative (CD45RO, CD39, CD25). Significant differences between groups were determined by ANOVA or Mann Whitney U tests.
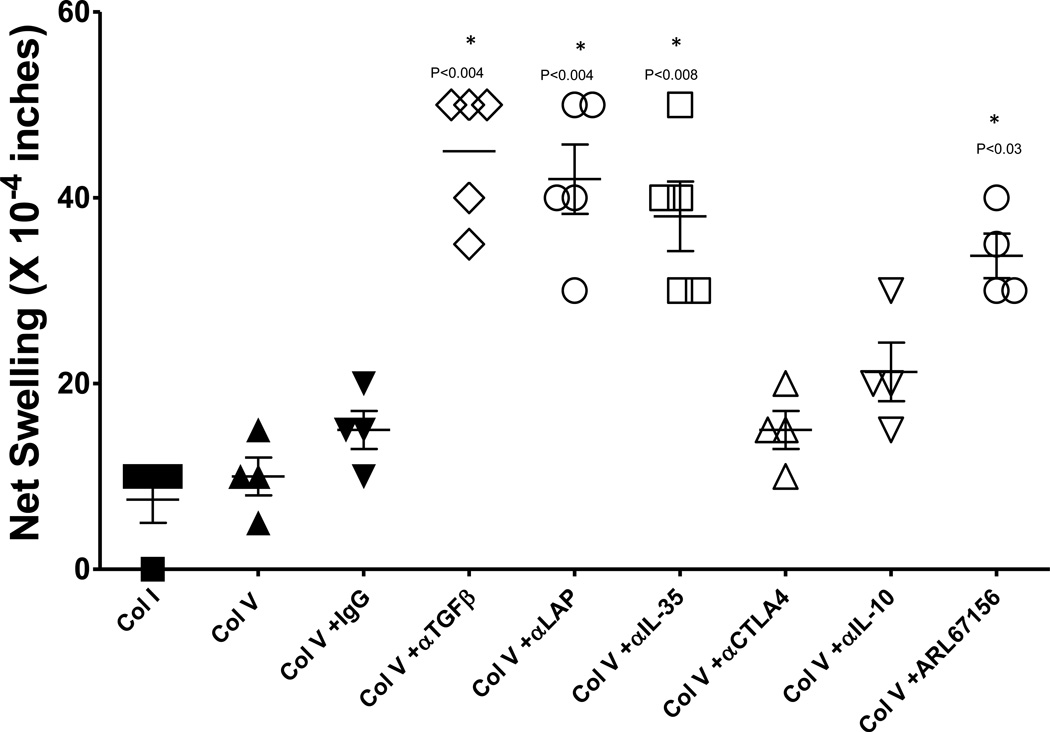
To define the mechanism by which the CD39+ and CD25+ Treg controls ColV reactivity in normal, healthy individuals, we investigated whether blockade of Treg associated cytokines or surface receptors could reveal a ColV response without the requirement for separation of regulatory from effector T cell subsets. Figure 3 shows that significant ColV responses were revealed in whole PBMCs from normal, healthy individuals when TGFβ (p<0.004), LAP (p<0.004) or IL-35 (p<0.008) was neutralized. We observed no significant increase in ColV responses when CTLA-4 was blocked, or when IL-10 was neutralized. ColV responses were also uncovered in whole PBMCs by the CD39 ATPase inhibitor, ARL67156 (2 hour pretreatment , 100uM) .
Figure 3. Uncovering Col V responses via TGFβ, IL-35 and CD39 inhibition.
In whole PBMCs from normal individuals, neutralizing antibodies to TGFβ (p<0.004), LAP (p<0.004), IL-35 (p<0.008) or chemical inhibition of CD39 activity (ARL67156, p<0.03) uncover Col V, responses. Neither CTLA-4 nor IL-10 caused a significant increase in the Col V response over that observed in whole PBMCs alone (P values not shown) or Col V plus IgG isotype (P values shown). Data are from 4–7 individuals. Significant differences between groups were determined by ANOVA or Mann Whitney U tests.
CD39+ T Cell depletion uncovers reactivity to select auto-antigens in healthy individuals
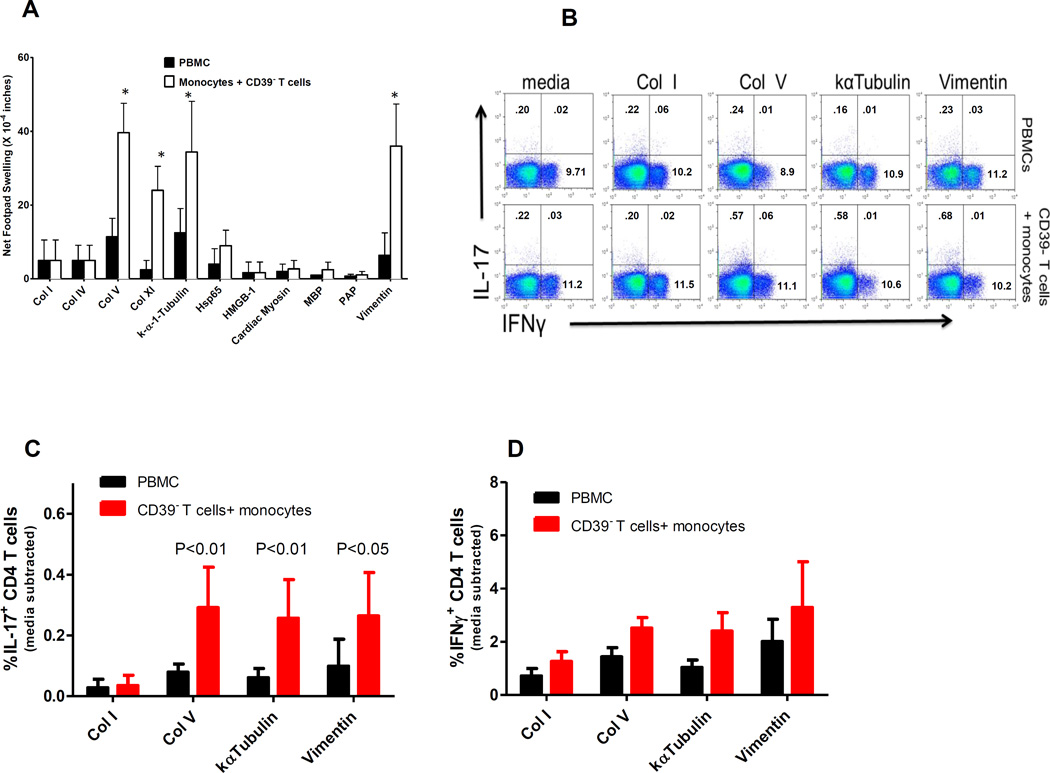
We next investigated whether by depleting CD39+ T cells, or by TGFβ neutralization, we could uncover T cell responses to other self proteins besides ColV. Figure 4A shows that like ColV, Col XI (the closely related minor collagen found associated with Col II in cartilage) stimulated strong tvDTH responses in CD39− T cell plus monocyte preparations from normal, healthy individuals. In addition, kα-1-tubulin and vimentin, targets of the autoimmune response in lung and heart transplant recipients with no homology to fibrillary collagens, also elicited strong responses. Certain self antigens associated with autoimmune disease, namely Col IV, HSP65, HMGB-1, cardiac myosin, MBP and PAP did not elicit significant responses. Based on the results from panel A, we investigated whether or not kα-1-tubulin and vimentin, like ColV, would exhibit enhanced IL-17 production when CD39+ T cells were removed. This was indeed the case. Panel 4B shows representative flow plots of IL-17 and IFNγ production in CD3+/CD4+ cells from whole PBMCs (top) and CD39- T cells plus monocyte groups (bottom). Similar to results observed with ColV in Figure 2, the corrected increase in IL-17+ CD4 T cells in response to kα-1-tubulin and vimentin was in the range of 0.2–0.6%; 40–60% of IL-17 producing CD4 T cells were also positive for TNFα, and 10–30% were positive for IL-22 production (data not shown). Quantified flow cytometry data indicates that ColV (p<0.01), kα-1-tubulin (p<0.01) and vimentin (p<0.05) all elicited an increased frequency of IL-17 producing CD4 T cells in CD39− T cells plus monocytes as compared to unseparated PBMCs (Figure 4C). While a trend towards increased IFNγ production in response to kα-1-tubulin and vimentin was observed, the differences from the Col I response did not reach statistical significance (p=0.14)(Figure 4D).
Figure 4.
CD39+ T cell depletion uncovers reactivity to select transplant associated proteins in normal healthy individuals. (A) Multiple proteins were examined to determine if CD39+ T cell depletion uncovered cellular immune responses to these antigens. Self-antigens commonly associated with autoimmune disease (Col IV, myelin basic protein, cardiac myosin), DAMP activity (HMGB1), or prostate tumor immunity (PAP) yielded no response over PBMC ‘background’. However, three self-antigens that have been associated with transplant-induced autoimmunity (Col V, kα-1-tubulin, and vimentin) all elicited a strong response. * Represents significance (p<0.05) vs PBMC- stimulated value. Data are from 5–11 individuals. (B) Removal of CD39+ T cells uncovers a population of ColV, kα-1-tubulin or vimentin specific IL-17 producing CD4 T cells. Representative flow plots from two individuals of IL-17 and IFNγ production from whole PBMCs or CD39− T cells plus monocytes treated overnight in the presence or absence of the indicated protein. Inset numbers represent observed percentage in each quadrant. (C) Quantified flow data from 5–7 normal, healthy individuals illustrating the corrected frequency of IL-17 (C) or IFNγ (D) positive CD4 T cells. Significant differences between groups (A-D) were determined by Mann Whitney U tests.
Col V, kα-1-tubulin and Vimentin specific cellular immune responses are present in non-human primates
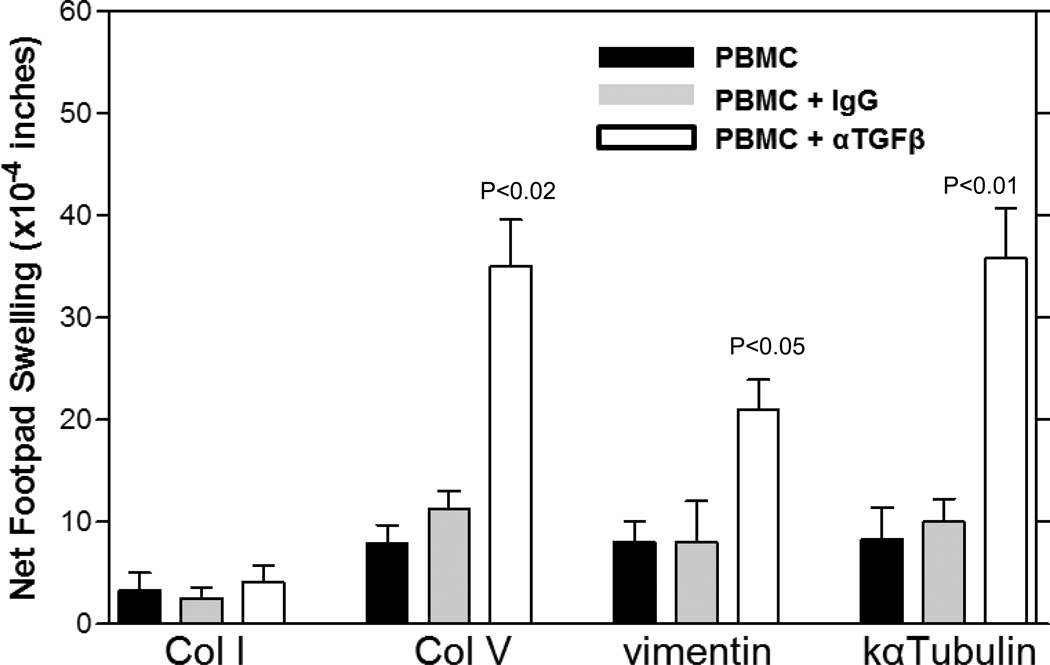
To ascertain whether TGFβ-regulated, pre-existing cellular immune responses to ColV, kα-1-tubulin and vimentin were evolutionarily conserved between humans and non-human primates, we used a TGFβ neutralization and tvDTH approach in PBMC of healthy, Rhesus Macaques (Figure 5). Whole PBMCs, PBMCs plus IgG control or PBMCs plus αTGFβ were mixed with antigen and injected into the footpads of CB17.SCID mice. ColV (p<0.02), kα-1-tubulin (p<0.01) and vimentin (p<0.05) all exhibited a significant increase in footpad swelling in the tvDTH assay (Figure 5). This result was not observed in Col I stimulated PBMC samples.
Figure 5.
Cellular immune responses to Col V, vimentin and kα-1-tubulin are conserved in non-human primates. PBMCs from blood of 5–9 Rhesus Macaques were attained and responses to Col I, Col V, kα-1-tubulin and vimentin were investigated using TGFβ (25ug) neutralization-uncovering techniques. A significant increase in swelling was observed with Col V (p<0.02), kα-1-tubulin (p<0.01) and Vimentin (p<0.05) when TGFβ was neutralized vs IgG treated controls. Data are from 5–11 animals/group. Significant differences between groups were determined by Mann Whitney U tests.
Cellular immune responses to ColV, Vimentin and kα-1-tubulin appear early in human lymphoid development
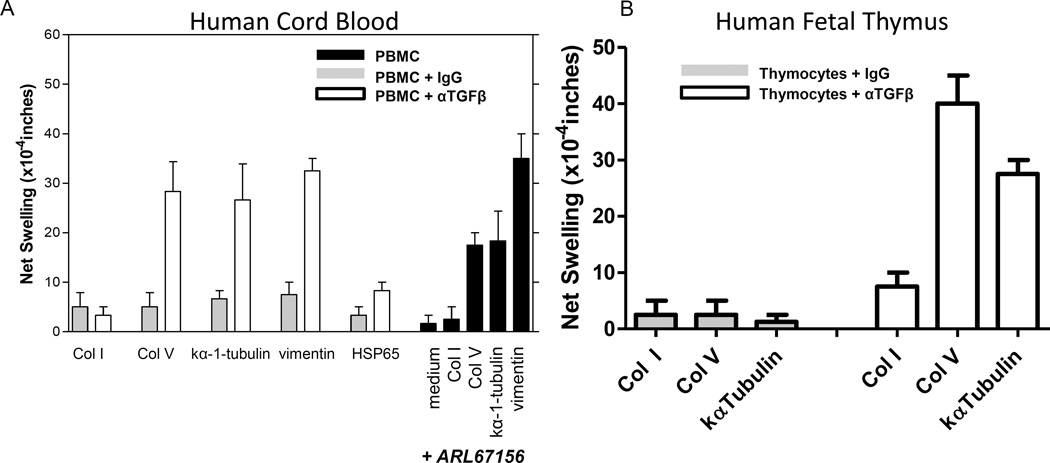
To investigate the ontogeny of antigen specific Th17 responses to Col V, kα-1-tubulin and vimentin, we first analyzed samples (n=3) of cryo-preserved CD34 depleted human cord blood using the tvDTH assay. In Figure 6A, we observed that while Col I, ColV, kα-1-tubulin, HSP65 and vimentin responses were low in cord blood, TGFβ neutralization revealed strong responses to ColV, kα-1-tubulin and vimentin. These responses were antigen-specific, in that no response to Col I and only a weak response to HSP65 were detected. Furthermore, treatment of cord blood cells with ARL67156 selectively uncovered responses to ColV, kα-1-tubulin and vimentin, suggesting that the cells mediating these responses in the cord blood were not only suppressed by TGFβ, but by downstream products of the CD39 ATPase.
Figure 6.
Cellular immune responses to Col V, vimentin and kα-1-tubulin are present in human cord blood and fetal thymus. (A) Commercially available, cryopreserved, CD34 depleted cord blood samples were attained from 3 different donors and responses to Col I, Col V, kα-1-tubulin and vimentin were investigated using TGFβ neutralization strategies or pharmacological inhibition of CD39 activity with ARL67156. A dramatic increase in swelling was observed with Col V, kα-1-tubulin and Vimentin when either TGFβ was neutralized or CD39 enzymatic activity was inhibited (ARL67156) vs IgG treated controls. (B) Fetal thymus samples (16 and 17 weeks) from two individuals were attained along with autologous splenic wedge. Thymocytes were isolated and mixed with T cell depleted splenocytes (for antigen presentation) and Col I, Col V, or kα-1-tubulin with or without TGFβ neutralization.
These results implied that pre-existing Th17 cells and the corresponding Tregs, specific for ColV, kα-1-tubulin and vimentin, had developed during in utero life. We obtained two samples of mid-gestation age (16–23 weeks) human thymus and spleen. We used T cell depleted autologous splenocytes as a source of APC and mixed them with unseparated thymocytes. No attempt was made to enrich for mature, single positive CD4+ or CD8+ cells. We observed no response to Col I, ColV or kα-1-tubulin in the unmodified tvDTH assay. In the presence of TGFβ neutralizing antibody, however, we observed a specific increase in the response to ColV and kα-1-tubulin, but not to Col I. We also examined whether there was antigen specific IL-17 production from fetal thymocytes in the absence of splenic APC. We observed an increase in IL-17 amongst single positive CD4 T cells from fetal thymocytes depleted of CD25 that were treated with ColV and kα-1-tubulin, but not Col I (figure S1).
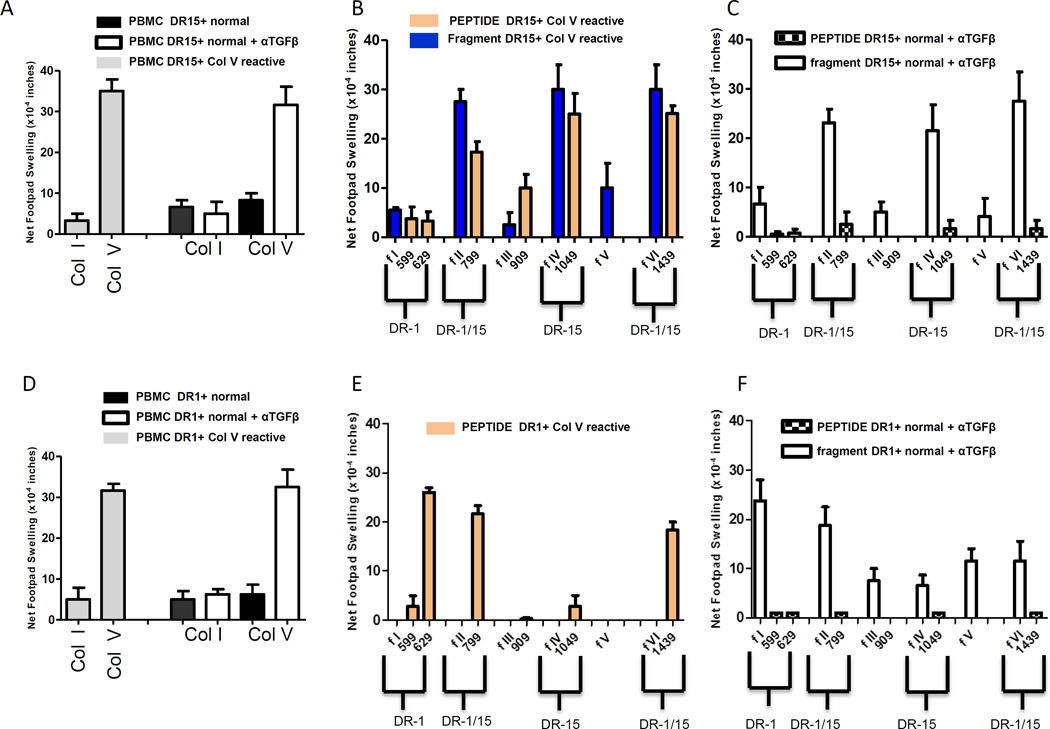
Th17 cells respond in HLA-DR restricted fashion to selected fragments of the ColV α1 chain, but not to synthetic 15mer peptides contained therein
We previously reported that ColV reactive patients who are HLA-DR-15, or who are DR15 negative but have received a DR15+ lung transplant, show strong cellular immune responses to DR-15 binding synthetic 15mer peptides of the collagenous region of Col V (α1), including peptide p1439, p1049 and p799 (39). Recently, we have designed and expressed portions of the α1 region of ColV in HEK 293 cells(40)( Figure S2). Of the six recombinant fragments of the collagenous region (peptides 551-1581), only fragment 1 contained both a DR-1 restricted (p629) and a DQ-restricted (p599) peptide sequence, while fragment 5 contained no known DR- or DQ binding peptide(40). We identified three normal, healthy individuals who were HLA-DR15+ and one who served as a HLA-DR1+ - DR15− control subject. In Figure 7, we investigated whether responses to HLA-DR restricted peptides or fragments of ColV containing these peptides could be revealed by TGFβ neutralization. In panel 7A, the ColV response revealed by TGFβ neutralization in normal, healthy DR15+ individuals (n=3) was similar in magnitude to that observed without TGFβ neutralization in DR15+ ColV reactive transplant patients (n=2). Unlike the ColV reactive patients, healthy DR15+ individuals failed to respond to any synthetic peptide (checkered bar), however, they did respond to fragments (2,4 and 6) containing the DR15 binding peptides. In contrast, the ColV-reactive DR15+ patients responded to the three peptides (p799, p1049 and p1439) predicted to bind DR15, as well as fragments 2, 4 and 6. In contrast, the DR-1 binding peptide p629, the DQ-binding peptides, p909 and p599, fragment 1 (containing peptides p599 & p629), fragment 3 (containing p909) and fragment 5 did not elicit a response (Figure 7B).
Figure 7.
Th17 cells respond in HLA-DR-restricted fashion to selected regions of the Col V chain, but not to synthetic 15mer peptides . (A) tvDTH responses to intact Col I or Col V by PBMCs from HLA-DR-15+ Col V reactive patients (grey), and by normal, healthy DR15+ individuals, in the absence (black) or presence of a TGFβ neutralizing antibody (white). (B) Responses of PBMC from DR-15+, Col V reactive patients (n=2) to DR-specific Col V-α-1 peptides (orange) or recombinant fragments (blue) of the collagenous domain of the α-1 V chain of Col V, with the indicated peptide contained therein. (C) Responses of PBMCs from normal, healthy DR-15+ individuals (n=3) in the presence of TGFβ neutralizing antibodies to the indicated Col V α1-peptide (checkered bar) or fragment (open bar). (D) Responses to intact Col I or Col V in whole PBMCs from a DR-1+ Col V reactive patient (grey), and by a normal, healthy DR1+ individual in the absence (black) or in the presence of a TGFβ neutralizing antibody (white). (E) Responses of PBMC from the DR-1+, Col V reactive patient, to DR-specific Col V-α-1 peptides (orange). Please note, fragments were not tested on this individual. (F) Responses of PBMC of the normal, healthy DR-1+ individual in the presence of TGFβ neutralizing antibody to the indicated Col V peptide (checkered bar) or fragment (open bar). All tvDTH experiments were repeated at least twice.
To investigate whether the restriction pattern was different in HLA-DR1+ normals, we compared PBMC of a DR1+ ColV reactive patient with a DR1+ healthy individual. In the patient, we observed that peptide p629 (DR1 specific) induced a strong response in the tvDTH assay, while the DR15-specific peptide, p1049 did not. The DQ-binding peptides p599 and p909, each stimulated a weak response. In control DR1+ PBMC, fragment 1, containing the DR1-specific peptide p629, which had failed to stimulate any response by DR15+ controls under TGFβ neutralizing conditions (Figure 7C), elicited a strong response. Fragment 2 containing the DR1/15 cross-reactive epitope p799, also elicited a strong response in the DR1+ healthy control but not fragments 4-containing the DR15-specific epitope, p1049 or 6, containing the DR1/15 cross-reactive epitope p1439 (Figure 7D-F). In the patient, we observed that peptide p629 (DR1 specific) induced a strong response in the tvDTH assay, while the DR15-specific peptide, p1049 did not. The DQ-binding peptides p599 and p909, each stimulated a weak response.
Discussion
Chronic rejection of lung and heart allografts has been associated with an unusual type of autoimmune response, in which antibody and T cell responses to kα-1-tubulin (lung), vimentin (heart) and ColV (lung and heart) arise in conjunction with the fibro-obliteration of airway and vascular spaces. In the lung, airway obliteration in both mouse and rat allograft models has been shown to depend on IL-17 (41, 42). However, the source of the IL-17 driving fibro-obliteration, whether from innate or adaptive T lymphocytes, or both, is still controversial (43).
In this study, we discovered a pre-existing Th17 response to ColV in humans, monkeys and mice, simply by Treg functional blockade or removal. The most straightforward way to demonstrate this phenomenon was by tvDTH assay. This bioassay relies on purinergic and leukotriene signaling between human and mouse leukocytes and readily detects human purinergic–receptor-dependent Th17 responses in transplant chronic rejection and atherosclerotic disease (11–15). The frequency of IL17+ CD4 T cells that we typically found by PMA/Ionomyocin stimulation, in media-only cultures of whole PBMC, or in T cells plus monocyte cultures after CD39+ T cell depletion, 0.2–0.7%, is well within the range of the frequency of IL-17 producing CD4 T cells observed by other groups in the healthy population (44). On top of this baseline response, after CD39+ T cell depletion, we observed an additional 0.3–0.4% of CD4 T cells producing IL-17 in response to ColV, kα-1-tubulin or vimentin, but not Col I (Figures 2 & 4). This response, not previously detected in healthy individuals, suggests that pre-existing Th17 cells specific for the three self antigens that have been thus far associated with autoimmunity after lung and heart transplantation are in similar frequency in peripheral blood. The similar frequency of ColV, kα-1-tubulin and vimentin responsive cells in healthy individuals is likely not due to a poly-reactive responding CD4 T cell, similar to natural, poly-reactive B cells responding to self-antigens (45, 46), as stimulation of fetal thymocytes with both ColV and kα-1-tubulin gave IL-17 responses greater than that observed with ColV or kα-1-tubulin alone (Figure S1).
Other common self antigens that have been associated with autoimmune disease including Col I (Scleroderma), Col IV (Goodpasture’s Syndrome), MBP (multiple sclerosis), cardiac myosin (auto-immune cardiomyopathy) or with an anti-prostate tumor response (PAP) were not included in immune surveillance by this pre-existing Th17 system, controlled by the actions of TGFβ (and IL-35) produced by a CD39+/CD25+ Treg. Cardiac Myosin has been identified by Fedoseyeva et al. as an autoantigen that triggers an immune response in certain murine heart transplant models(47). Surprisingly, no pre-existing Th17 response to this autoantigen was observed. We attribute this to the predominantly Th1 nature of the anti-Cardiac Myosin response, since Th1 responses may require a dendritic cell as APC, rather than the monocyte APC used in the tvDTH experiment in Figure 4.
Since ColV responses could be revealed by a specific inhibitor of CD39-ATPase activity, the Th17 mediated responses to these self antigens is also likely subject to control by adenosine, a product of the CD39 catalyzed breakdown of extracellular ATP. In addition, the Th17 cell specific for ColV, kα-1-tubulin and vimentin observed throughout this report concomitantly produced the Th17 associated inflammatory cytokine TNFα (60–80%), as well as IL-22 (20–30%), a cytokine implicated in the repair of damaged epithelial surfaces. Both of these cytokines contributed significantly to the tvDTH footpad swelling response (Figure 1). The TNFα/IL-22 findings are similar to that reported in conventional Th17 cells (48–54), and supports the idea that Tregs and Th17 cells may be allies in protecting epithelial surfaces
In the presence of anti-TGFβ antibody, healthy HLA-DR15+ or HLA-DR1+ individuals had no reactivity to synthetic peptides but show reactivity to intact ColV, and to those α1(V) fragments containing the appropriate HLA-DR restricted peptides (Figure 7). One explanation for this finding is that the ColV specific Th17 cells we observed in transplant and coronary artery disease patients are memory Th17 cells, of higher affinity than the ones revealed through regulatory T cell depletion or functional blockade in normal, healthy individuals. ColV– reactive memory Th17 cells in transplant and coronary artery disease patients also likely require a lower threshold of activation vs the antigen-specific, pre-existing Th17 cells we observed in healthy individuals, thereby enabling such T cells to respond to the peptide alone. Alternatively, the pre-existing Th17 cells may have similar affinity for peptide/MHC as do Th17 cells from ColV reactive patients, which do respond to synthetic DR-binding peptides of α1(V). However, in healthy controls, peptides alone provide insufficient stimulation of the antigen presenting cell, resulting in a lack of secondary signal to the effector T cell. This secondary signal may be induced in the APC by the T cell in the pathologic setting after peptide recognition that would bypass this requirement.
The data in Figure 6 strongly support the idea that the Th17 cells we observe in normal, healthy individuals are thymically differentiated, pre-existing Th17 cells as these cells are present in fetal thymus as well as human cord blood (Figure 6). We hypothesize that pre-existing Th17 cells may recognize ColV, kα-1-tubulin or vimentin transiently exposed during normal cycles of injury/repair around airway and vascular structures. As such, they would not chronically be exposed to their target self antigens, which are subsequently sequestered with major collagen I (Col V) or collagen II (Col XI) fibrils or within cells (kα-1-tubulin and vimentin) during tissue repair.
Zhang et al (55) have identified effector T cells from human cord blood, including Th1, Th2 and Th17. In this report, it was observed that while effector function for Th1 and Th2 cells was observed without in vitro culture, Th17 cells required three days of culture in the presence of Th17 polarizing conditions before they were detectable. This contrasts with our report as we were able to identify ColV, kα-1-tubulin and vimentin responses in cord blood through TGFβ neutralization, using the overnight tvDTH assay with no in vitro culture. However, it should be noted that in order to detect pre-existing Th17 responses in overnight cultures, PMA/Ionomyocin treatment was required.
The pre-existing Th17 responses in CBA mice (Figure 1) and in Rhesus macaques (Figure 5), indicate an evolutionarily conserved function of responses to ColV, k-α-1-tubulin and vimentin. In a previous study, we found the human HLA-DQ2 and DQ6, and the mouse DQ equivalent IAb bound similar α1(V) peptides, p599 and p909, suggesting that the selection of epitopes for DQ-restricted responses to Col V by CD4 T cells is also conserved (39). One possibility is that the maintenance and homeostasis of epithelial or endothelial cell barrier layers alluded to above requires the thymus to produce MHCII-restricted, innate-like T cells. Another is that preservation of commensal micro-organisms needed for proper epithelial function and immune development, requires their presence at epithelial interfaces.
In either case, we hypothesize that Th17-mediated pathologies may develop when a pre-existing Treg-Th17 balance is lost. A loss of balance may result from chronic exposure of these antigens in the extra-cellular matrix during repeated infections, atherosclerosis (40), or the ischemia-reperfusion injury of organ procurement and transplant surgery(38). A high ratio of extracellularATP:adenosine, along with standard immunosuppression, may inhibit CD39+ Treg function and allow pre-existing Th17 cells to escape from regulatory control. This might select for higher affinity, peripherally-induced Th17 clones that are peptide-reactive. This possible connection is still unproven. The alternative idea is that pathogenic, induced Th17 responses to these self antigens seen after transplant is completely unrelated to disruption of a pre-existing Treg:Th17 homeostasis (56).
Recently, tissue-resident CD4 T cells in the donor organ have been shown to play a key role in triggering host T follicular helper and B cell responses to vimentin(57). If the pre-existing Treg:Th17 balance of both the donor and the recipient toward ColV, kα-1-tubulin and vimentin, are critical for graft success, and if some DR antigens are better than others at presenting peptides of these 3, then the particular DR or DQ antigens expressed by the donor organ may dictate the success of the transplant (39).
In conclusion, we have identified a novel system of pre-existing Th17 development present at the time of, and well before, birth. Unlike the thymically derived iNK-T cell, the pre-existing Th17 cell described herein depends on polymorphic MHC-II restricted antigen presentation, classically associated with adaptive immunity, while retaining certain features typically associated with innate immunity. Besides its implications for heart and lung transplantation, the results in human, monkey and mouse provide a self-antigen specificity for a subset of pre-existing Th17 cells previously described as thymically-differentiated T cells that respond rapidly to oral candidiasis, and protect upper airway, lung and gut mucosa (58, 59).
Supplementary Material
Figure S1: Agonist specific IL-17 production from human thymus is revealed when CD25+ T cells are removed. Groups of thymocytes or CD25 depleted thymocytes from a 19 week human fetus were stimulated with either Col I, ColV, kα-1-tubulin or in combination. (A) Flow plots gated on single positive CD3+/CD4+ T cells representing IL-17 and IFNγ production in groups of thymocytes or CD25 depleted thymocytes after overnight stimulation with the indicated antigens. Inset numbers represent the frequencies of IL-17 and IFNγ positive cells gated from single positive CD3+/CD4+ T cells. (B) Quantified frequencies of IL-17+ CD4 T cells from panel A, with the media frequency of IL-17+ populations subtracted.
Figure S2: Peptide epitopes and recombinant fragments of ColV. Peptides of ColV (15mers) representing the determined HLA-DR binding epitopes. DR1 and DR15 binding epitopes are contained within both fragments 2 and 6. DR15 only binding epitopes are contained in fragment 4. A DR1 only binding epitope is contained in fragment 1. The less immunogenic DQ2/DQ6/I-Ab epitopes are contained within fragments 1 and 3. Fragment 5 contains no known DR/DQ epitopes of col Vα1.
Acknowledgments
The authors wish to thank the laboratory of Sam Gellman for the production of ColV (α1) peptides and for helpful suggestions on the manuscript. JAS designed and performed experiments, analyzed the data and wrote the manuscript. EJG designed and performed experiments, analyzed data and aided in manuscript preparation. SH, MAP, VVA, ACP, JFK and MEB performed experiments and aided in the preparation of the manuscript. DBK and DSW aided in the design of experiments. DSG designed experiments and aided in manuscript preparation. WJB aided in the design of all experiments, analyzed data and wrote the manuscript. This work was supported by NIH grants PO1AI084853 (JAS, EJG, VVA, DSW, ACP, DSG, WJB), U01AI102456-04 (DBK, WJB), T32GMO81061 (MEB), TL1TR000429 (MEB), UL1TROOO427 (MEB), and by the EU-sponsored One Study (WJB).
Abbreviations
- APC
antigen-presenting cell
- Col V
collagen type V
- α1V
Col V triple helical region
- CAD
coronary artery disease
- HFTR
Human Fetal Tissue Repository
- PBMC
peripheral blood mononuclear cell
- tvDTH
Trans-vivo delayed type hypersensitivity
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. David S. Wilkes is a co-founder of ImmuneWorks, Inc., a biotechnology company involved in designing therapeutics for various forms of lung diseases. The other authors have no conflicts of interest to disclose.
References
- 1.Kotloff RM, Thabut G. Lung transplantation. Am J Respir Crit Care Med. 2011;184:159–171. doi: 10.1164/rccm.201101-0134CI. [DOI] [PubMed] [Google Scholar]
- 2.Trulock EP. Lung transplantation. Am J Respir Crit Care Med. 1997;155:789–818. doi: 10.1164/ajrccm.155.3.9117010. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan JA, Adams AB, Burlingham WJ. The emerging role of TH17 cells in organ transplantation. Transplantation. 2014;97:483–489. doi: 10.1097/TP.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 4.Martin JC, Baeten DL, Josien R. Emerging role of IL-17 and Th17 cells in systemic lupus erythematosus. Clin Immunol. 2014;154:1–12. doi: 10.1016/j.clim.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Leipe J, Grunke M, Dechant C, Reindl C, Kerzendorf U, Schulze-Koops H, Skapenko A. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62:2876–2885. doi: 10.1002/art.27622. [DOI] [PubMed] [Google Scholar]
- 6.Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, Melendez AJ, McInnes IB. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010;184:3336–3340. doi: 10.4049/jimmunol.0903566. [DOI] [PubMed] [Google Scholar]
- 7.Benham H, Norris P, Goodall J, Wechalekar MD, FitzGerald O, Szentpetery A, Smith M, Thomas R, Gaston H. Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis research & therapy. 2013;15:R136. doi: 10.1186/ar4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimoto S, Kotani H, Tsuruta S, Shimizu N, Ito M, Shichita T, Morita R, Takahashi H, Amagai M, Yoshimura A. Th17 cells carrying TCR recognizing epidermal autoantigen induce psoriasis-like skin inflammation. J Immunol. 2013;191:3065–3072. doi: 10.4049/jimmunol.1300348. [DOI] [PubMed] [Google Scholar]
- 9.Jadidi-Niaragh F, Mirshafiey A. Th17 cell, the new player of neuroinflammatory process in multiple sclerosis. Scand J Immunol. 2011;74:1–13. doi: 10.1111/j.1365-3083.2011.02536.x. [DOI] [PubMed] [Google Scholar]
- 10.Lovett-Racke AE, Yang Y, Racke MK. Th1 versus Th17: are T cell cytokines relevant in multiple sclerosis? Biochimica et biophysica acta. 2011;1812:246–251. doi: 10.1016/j.bbadis.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, Gopalakrishnan B, Cai J, Brand DD, Yoshida S, Cummings OW, Wilkes DS. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. The Journal of clinical investigation. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dart ML, Jankowska-Gan E, Huang G, Roenneburg DA, Keller MR, Torrealba JR, Rhoads A, Kim B, Bobadilla JL, Haynes LD, Wilkes DS, Burlingham WJ, Greenspan DS. Interleukin-17-dependent autoimmunity to collagen type V in atherosclerosis. Circulation research. 2010;107:1106–1116. doi: 10.1161/CIRCRESAHA.110.221069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan JA, Jankowska-Gan E, Shi L, Roenneburg D, Hegde S, Greenspan DS, Wilkes DS, Denlinger LC, Burlingham WJ. Differential Requirement for P2X7R Function in IL-17 Dependent vs. IL-17 Independent Cellular Immune Responses. Am J Transplant. 2014 doi: 10.1111/ajt.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, D'Addio F, Mfarrej B, Donnarumma M, Habicht A, Clarkson MR, Iacomini J, Glimcher LH, Sayegh MH, Ansari MJ. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. The Journal of experimental medicine. 2008;205:3133–3144. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bobadilla JL, Love RB, Jankowska-Gan E, Xu Q, Haynes LD, Braun RK, Hayney MS, Munoz del Rio A, Meyer K, Greenspan DS, Torrealba J, Heidler KM, Cummings OW, Iwata T, Brand D, Presson R, Burlingham WJ, Wilkes DS. Th-17, monokines, collagen type V, and primary graft dysfunction in lung transplantation. American journal of respiratory and critical care medicine. 2008;177:660–668. doi: 10.1164/rccm.200612-1901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun RK, Molitor-Dart M, Wigfield C, Xiang Z, Fain SB, Jankowska-Gan E, Seroogy CM, Burlingham WJ, Wilkes DS, Brand DD, Torrealba J, Love RB. Transfer of tolerance to collagen type V suppresses T-helper-cell-17 lymphocyte-mediated acute lung transplant rejection. Transplantation. 2009;88:1341–1348. doi: 10.1097/TP.0b013e3181bcde7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada Y, Sekine Y, Yoshida S, Yasufuku K, Petrache I, Benson HL, Brand DD, Yoshino I, Wilkes DS. Type V collagen-induced oral tolerance plus low-dose cyclosporine prevents rejection of MHC class I and II incompatible lung allografts. J Immunol. 2009;183:237–245. doi: 10.4049/jimmunol.0804028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bharat A, Saini D, Steward N, Hachem R, Trulock EP, Patterson GA, Meyers BF, Mohanakumar T. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. The Annals of thoracic surgery. 2010;90:1094–1101. doi: 10.1016/j.athoracsur.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180:4487–4494. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiriveedhi V, Angaswamy N, Weber J, Mohanakumar T. Lipid raft facilitated ligation of K-alpha1-tubulin by specific antibodies on epithelial cells: Role in pathogenesis of chronic rejection following human lung transplantation. Biochemical and biophysical research communications. 2010;399:251–255. doi: 10.1016/j.bbrc.2010.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahesh B, Leong HS, McCormack A, Sarathchandra P, Holder A, Rose ML. Autoantibodies to vimentin cause accelerated rejection of cardiac allografts. The American journal of pathology. 2007;170:1415–1427. doi: 10.2353/ajpath.2007.060728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jurcevic S, Ainsworth ME, Pomerance A, Smith JD, Robinson DR, Dunn MJ, Yacoub MH, Rose ML. Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac transplantation. Transplantation. 2001;71:886–892. doi: 10.1097/00007890-200104150-00011. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O'Farrelly C, Tubridy N, Mills KH. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 24.Grant CR, Liberal R, Holder BS, Cardone J, Ma Y, Robson SC, Mieli-Vergani G, Vergani D, Longhi MS. Dysfunctional CD39(POS) regulatory T cells and aberrant control of T-helper type 17 cells in autoimmune hepatitis. Hepatology. 2014;59:1007–1015. doi: 10.1002/hep.26583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant CR, Liberal R, Mieli-Vergani G, Vergani D, Longhi MS. Regulatory T-cells in autoimmune diseases: challenges, controversies and--yet--unanswered questions. Autoimmun Rev. 2015;14:105–116. doi: 10.1016/j.autrev.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Longhi MS, Moss A, Bai A, Wu Y, Huang H, Cheifetz A, Quintana FJ, Robson SC. Characterization of human CD39+ Th17 cells with suppressor activity and modulation in inflammatory bowel disease. PloS one. 2014;9:e87956. doi: 10.1371/journal.pone.0087956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. The Journal of experimental medicine. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell'Acqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 29.Ohta A, Sitkovsky M. Extracellular adenosine-mediated modulation of regulatory T cells. Frontiers in immunology. 2014;5:304. doi: 10.3389/fimmu.2014.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ernst PB, Garrison JC, Thompson LF. Much ado about adenosine: adenosine synthesis and function in regulatory T cell biology. J Immunol. 2010;185:1993–1998. doi: 10.4049/jimmunol.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linden J, Cekic C. Regulation of lymphocyte function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32:2097–2103. doi: 10.1161/ATVBAHA.111.226837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joosten LA. Excessive interleukin-1 signaling determines the development of Th1 and Th17 responses in chronic inflammation. Arthritis Rheum. 2010;62:320–322. doi: 10.1002/art.27242. [DOI] [PubMed] [Google Scholar]
- 33.Hanidziar D, Koulmanda M. Inflammation and the balance of Treg and Th17 cells in transplant rejection and tolerance. Curr Opin Organ Transplant. 2010;15:411–415. doi: 10.1097/MOT.0b013e32833b7929. [DOI] [PubMed] [Google Scholar]
- 34.Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009;21:274–280. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 35.Chung BH, Oh HJ, Piao SG, Sun IO, Kang SH, Choi SR, Park HS, Choi BS, Choi YJ, Park CW, Kim YS, Cho ML, Yang CW. Higher infiltration by Th17 cells compared with regulatory T cells is associated with severe acute T-cell-mediated graft rejection. Exp Mol Med. 2011;43:630–637. doi: 10.3858/emm.2011.43.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez DS, Jankowska-Gan E, Haynes LD, Leverson G, Munoz A, Heisey D, Sollinger HW, Burlingham WJ. Immune regulation and graft survival in kidney transplant recipients are both enhanced by human leukocyte antigen matching. Am J Transplant. 2004;4:537–543. doi: 10.1111/j.1600-6143.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 37.Jankowska-Gan E, Hegde S, Burlingham WJ. Trans-vivo Delayed Type Hypersensitivity Assay for Antigen Specific Regulation. Journal of visualized experiments : JoVE. 2013 doi: 10.3791/4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida S, Haque A, Mizobuchi T, Iwata T, Chiyo M, Webb TJ, Baldridge LA, Heidler KM, Cummings OW, Fujisawa T, Blum JS, Brand DD, Wilkes DS. Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am J Transplant. 2006;6:724–735. doi: 10.1111/j.1600-6143.2006.01236.x. [DOI] [PubMed] [Google Scholar]
- 39.Keller MR, Haynes LD, Jankowska-Gan E, Sullivan JA, Agashe VV, Burlingham SR, Burlingham WJ. Epitope Analysis of the Collagen Type V-Specific T Cell Response in Lung Transplantation Reveals an HLA-DRB1*15 Bias in Both Recipient and Donor. PloS one. 2013;8:e79601. doi: 10.1371/journal.pone.0079601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park AC, Huang G, Jankowska-Gan E, Massoudi D, Kernien JF, Vignali DA, Sullivan JA, Wilkes DS, Burlingham WJ, Greenspan DS. Mucosal Administration of Collagen V Ameliorates the Atherosclerotic Plaque Burden by Inducing Interleukin 35-dependent Tolerance. The Journal of biological chemistry. 2016;291:3359–3370. doi: 10.1074/jbc.M115.681882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDyer JF. Human and murine obliterative bronchiolitis in transplant. Proc Am Thorac Soc. 2007;4:37–43. doi: 10.1513/pats.200605-107JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakagiri T, Inoue M, Morii E, Minami M, Sawabata N, Utsumi T, Kadota Y, Ideguchi K, Tokunaga T, Okumura M. Local IL-17 production and a decrease in peripheral blood regulatory T cells in an animal model of bronchiolitis obliterans. Transplantation. 2010;89:1312–1319. doi: 10.1097/TP.0b013e3181d8ea16. [DOI] [PubMed] [Google Scholar]
- 43.Wu Q, Gupta PK, Suzuki H, Wagner SR, Zhang C, Cummings OW, Fan L, Kaplan MH, Wilkes DS, Shilling RA. CD4 T Cells but Not Th17 Cells Are Required for Mouse Lung Transplant Obliterative Bronchiolitis. Am J Transplant. 2015;15:1793–1804. doi: 10.1111/ajt.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60:1647–1656. doi: 10.1002/art.24568. [DOI] [PubMed] [Google Scholar]
- 45.Porcheray F, DeVito J, Yeap BY, Xue L, Dargon I, Paine R, Girouard TC, Saidman SL, Colvin RB, Wong W, Zorn E. Chronic humoral rejection of human kidney allografts associates with broad autoantibody responses. Transplantation. 2010;89:1239–1246. doi: 10.1097/TP.0b013e3181d72091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Porcheray F, DeVito J, Helou Y, Dargon I, Fraser JW, Nobecourt P, Ferdman J, Germana S, Girouard TC, Kawai T, Saidman SL, Wong W, Colvin RB, Leguern C, Zorn E. Expansion of polyreactive B cells cross-reactive to HLA and self in the blood of a patient with kidney graft rejection. Am J Transplant. 2012;12:2088–2097. doi: 10.1111/j.1600-6143.2012.04053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fedoseyeva EV, Zhang F, Orr PL, Levin D, Buncke HJ, Benichou G. De novo autoimmunity to cardiac myosin after heart transplantation and its contribution to the rejection process. J Immunol. 1999;162:6836–6842. [PubMed] [Google Scholar]
- 48.Moore-Connors JM, Fraser R, Halperin SA, Wang J. CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 responses and genital tract inflammation upon intracellular Chlamydia muridarum infection. J Immunol. 2013;191:3430–3439. doi: 10.4049/jimmunol.1301136. [DOI] [PubMed] [Google Scholar]
- 49.Pandiyan P, Conti HR, Zheng L, Peterson AC, Mathern DR, Hernandez-Santos N, Edgerton M, Gaffen SL, Lenardo MJ. CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity. 2011;34:422–434. doi: 10.1016/j.immuni.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Q, Hu Y, Howard OM, Oppenheim JJ, Chen X. In vitro generated Th17 cells support the expansion and phenotypic stability of CD4(+)Foxp3(+) regulatory T cells in vivo. Cytokine. 2014;65:56–64. doi: 10.1016/j.cyto.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li CR, Mueller EE, Bradley LM. Islet antigen-specific Th17 cells can induce TNF-alpha-dependent autoimmune diabetes. J Immunol. 2014;192:1425–1432. doi: 10.4049/jimmunol.1301742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Hamburg JP, Asmawidjaja PS, Davelaar N, Mus AM, Colin EM, Hazes JM, Dolhain RJ, Lubberts E. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2011;63:73–83. doi: 10.1002/art.30093. [DOI] [PubMed] [Google Scholar]
- 53.Kim CJ, McKinnon LR, Kovacs C, Kandel G, Huibner S, Chege D, Shahabi K, Benko E, Loutfy M, Ostrowski M, Kaul R. Mucosal Th17 cell function is altered during HIV infection and is an independent predictor of systemic immune activation. J Immunol. 2013;191:2164–2173. doi: 10.4049/jimmunol.1300829. [DOI] [PubMed] [Google Scholar]
- 54.Chen X, Oppenheim JJ. Th17 cells and Tregs: unlikely allies. Journal of leukocyte biology. 2014 doi: 10.1189/jlb.1213633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X, Mozeleski B, Lemoine S, Deriaud E, Lim A, Zhivaki D, Azria E, Le Ray C, Roguet G, Launay O, Vanet A, Leclerc C, Lo-Man R. CD4 T cells with effector memory phenotype and function develop in the sterile environment of the fetus. Sci Transl Med. 2014;6:238ra272. doi: 10.1126/scitranslmed.3008748. [DOI] [PubMed] [Google Scholar]
- 56.Burlingham W, Wilkes DS, Sullivan JA. Why is the patient out of breath? Collagen V(alpha1) and K-alpha1-tubulin take center stage in lung transplantation. Am J Transplant. 2014;14:2201–2203. doi: 10.1111/ajt.12910. [DOI] [PubMed] [Google Scholar]
- 57.Harper IG, Ali JM, Harper SJ, Wlodek E, Alsughayyir J, Negus MC, Qureshi MS, Motalleb-Zadeh R, Saeb-Parsy K, Bolton EM, Bradley JA, Clatworthy MR, Conlon TM, Pettigrew GJ. Augmentation of Recipient Adaptive Alloimmunity by Donor Passenger Lymphocytes within the Transplant. Cell Rep. 2016;15:1214–1227. doi: 10.1016/j.celrep.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marks BR, Nowyhed HN, Choi JY, Poholek AC, Odegard JM, Flavell RA, Craft J. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nature immunology. 2009;10:1125–1132. doi: 10.1038/ni.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Massot B, Michel ML, Diem S, Ohnmacht C, Latour S, Dy M, Eberl G, Leite-de-Moraes MC. TLR-induced cytokines promote effective proinflammatory natural Th17 cell responses. J Immunol. 2014;192:5635–5642. doi: 10.4049/jimmunol.1302089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Agonist specific IL-17 production from human thymus is revealed when CD25+ T cells are removed. Groups of thymocytes or CD25 depleted thymocytes from a 19 week human fetus were stimulated with either Col I, ColV, kα-1-tubulin or in combination. (A) Flow plots gated on single positive CD3+/CD4+ T cells representing IL-17 and IFNγ production in groups of thymocytes or CD25 depleted thymocytes after overnight stimulation with the indicated antigens. Inset numbers represent the frequencies of IL-17 and IFNγ positive cells gated from single positive CD3+/CD4+ T cells. (B) Quantified frequencies of IL-17+ CD4 T cells from panel A, with the media frequency of IL-17+ populations subtracted.
Figure S2: Peptide epitopes and recombinant fragments of ColV. Peptides of ColV (15mers) representing the determined HLA-DR binding epitopes. DR1 and DR15 binding epitopes are contained within both fragments 2 and 6. DR15 only binding epitopes are contained in fragment 4. A DR1 only binding epitope is contained in fragment 1. The less immunogenic DQ2/DQ6/I-Ab epitopes are contained within fragments 1 and 3. Fragment 5 contains no known DR/DQ epitopes of col Vα1.