Abstract
Organisms are often capable of modifying their development to better suit their environment. Under adverse conditions, the nematode Caenorhabditis elegans develops into a stress resistant alternative larval stage called dauer. The dauer stage is likely the primary survival stage for C. elegans in nature. Large-scale tissue remodeling during dauer conveys resistance to harsh environments in addition to behavioral changes. The environmental and genetic regulation of the decision to enter dauer has been extensively studied. However, less is known about the mechanisms regulating tissue remodeling. Changes to the cuticle and suppression of feeding in dauers lead to an increased resistance to external stressors. Meanwhile reproductive development arrests during dauer while preserving the ability to reproduce once favorable environmental conditions return. Dramatic remodeling of neurons, glia, and muscles during dauer likely facilitate dauer-specific behaviors. Dauer-specific pulsation of the excretory duct likely mediates a response to osmotic stress. The power of C. elegans genetics has uncovered some of the molecular pathways regulating dauer tissue remodeling. In addition to genes that regulate single remodeling events, several mutants result in pleiotropic defects in dauer remodeling. This review details the individual aspects of morphological changes that occur during dauer formation and discusses molecular mechanisms regulating these processes. The dauer stage provides us with an excellent model for understanding phenotypic plasticity and remodeling from the individual cell to an entire animal.
Graphical abstract
The dauer stage of C. elegans undergoes large-scale remodeling in several tissue types and is a model of phenotypic plasticity
INTRODUCTION
All organisms must cope with changing and adverse environmental conditions. Phenotypic plasticity is the ability of a single genotype to produce alternative phenotypes in response to the environment and is considered a driving force in evolution.1 While usually thought to be adaptive, inappropriate stress-induced phenotypic plasticity may lead to chronic pathologies. For example, chronic stress in mammals may cause neuroanatomical changes in brain regions associated with memory and fear processing.2 Cellular remodeling in response to stress is widespread.
Polyphenisms are types of plasticity wherein two or more distinct phenotypes may arise from a single genotype based on environmental inputs.1 Polyphenisms can entail extensive remodeling of various tissues. Among animals, invertebrates display some of the most remarkable examples of large-scale environmentally induced tissue remodeling. For example, the desert locust, Schistocerca gregaria, can alternate between distinct solitary and gregarious phases based on mechanically transmitted population density cues.3 Caste-formation in the honey bee, Apis mellifera, is due to dietary input during larval development leading to substantial differences in morphology and behavior between workers and queens.4,5
The cellular remodeling that occurs in these and other large-scale polyphenisms are extensive and ripe for inquiry. What are the molecular mechanisms controlling individual remodeling events? Are these mechanisms unique to the polyphenism or recycled from other non-environmentally induced developmental events? How are remodeling events in individual tissue types coordinated to produce a unique, but coherent phenotype suited to its environment?
THE C. ELEGANS DAUER STAGE IS A MODEL POLYPHENISM
The nematode Caenorhabditis elegans is an outstanding model for developmental genetics. It is easily reared on Escherichia coli and can complete one generation in three days.6 C. elegans is transparent, and the cell lineage is described.7,8 The complete connectome of the 302 neurons in the adult hermaphrodite is described allowing investigations into the neuroanatomical basis of behaviors at the level of a single cell.9 C. elegans is amenable to several genetic techniques including transformation, RNAi and genome editing.10–12
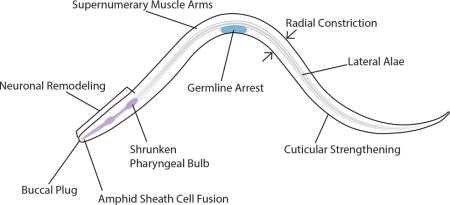
C. elegans displays a stress-induced developmental stage called dauer wherein several tissues undergo extensive remodeling to produce a stress-resistant and long-lived alternative developmental larva. In the presence of plentiful food and a low nematode population density, C. elegans develops through four larval stages before entering the adult reproductive stage. However, under specific adverse environmental conditions, C. elegans enters into a stress-resistant dauer developmental stage (Figure 1).13 Dauers show several differences from non-dauers that are immediately apparent under a dissecting microscope. Dauers are thinner than non-dauer L3s due to a general radial shrinkage of the body. Dauers suppress the pharyngeal pumping required for ingestion of bacterial food. They also tend to lie motionless but can be readily stimulated to move. The remodeling of dauers provide a tractable model for understanding the molecular mechanisms regulating remodeling of individual tissues events and how signaling between tissue types is coordinated to achieve a unique developmental stage.
Figure 1. The life cycle of C. elegans.
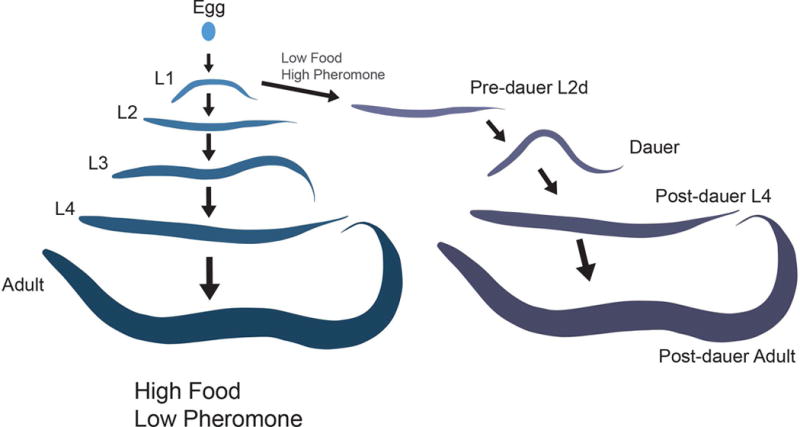
In an environment of plentiful food and low dauer pheromone, C. elegans molts through four larval stages followed by the reproductive adult. Under conditions of low levels of food and high levels of pheromone (signaling population density) during L1 they will enter into a developmentally arrested dauer stage. Following a return to favorable environmental conditions, they will recover from dauer and resume development into a reproductive adult.
The term dauer translates as ‘enduring’ from German. Indeed, C. elegans dauers are non-feeding and yet can survive for months whereas non-dauers live for about two weeks.14 One of the most common techniques for separating non-dauers from dauers is exposure to 1% sodium dodecyl sulfate (SDS) for 30 minutes.15 Non-dauers are killed within minutes whereas dauers appear completely unaffected. Preliminary data from our lab suggests that wild-type dauers are capable of surviving exposure to 10% SDS (K.F. unpublished). Several anatomical and behavioral changes occur during entry into dauer that allows animals to survive adverse conditions. For example, the survival to SDS is likely due to a combination of the cessation of feeding and ultrastructural changes in the cuticle.15
In addition to the absence of feeding, dauers display behaviors unique from non-dauers. On a Petri dish, non-dauers typically move continuously searching for food or mates. In contrast, dauers are frequently immobile. However, dauers are sensitive to touch and readily move following physical stimulation. Most noticeable among dauer-specific behaviors is “nictation” wherein a dauer will climb elevated objects, raise its body into the air and stand on its tail (Figure 2).15,16 In addition to individual nictating dauers, they will occasionally form undulating swarms containing numerous dauers (Box 1). Nictation is thought to serve as a dispersal strategy; by elevating its body above the ground, C. elegans can reduce surface tension and increase the possibility it will be picked up by a passing arthropod. Both experimental data and the association of C. elegans dauers with arthropods in nature support this dispersal hypothesis. 16,17
Figure 2. Nictation and swarming are dauer-specific behaviors.
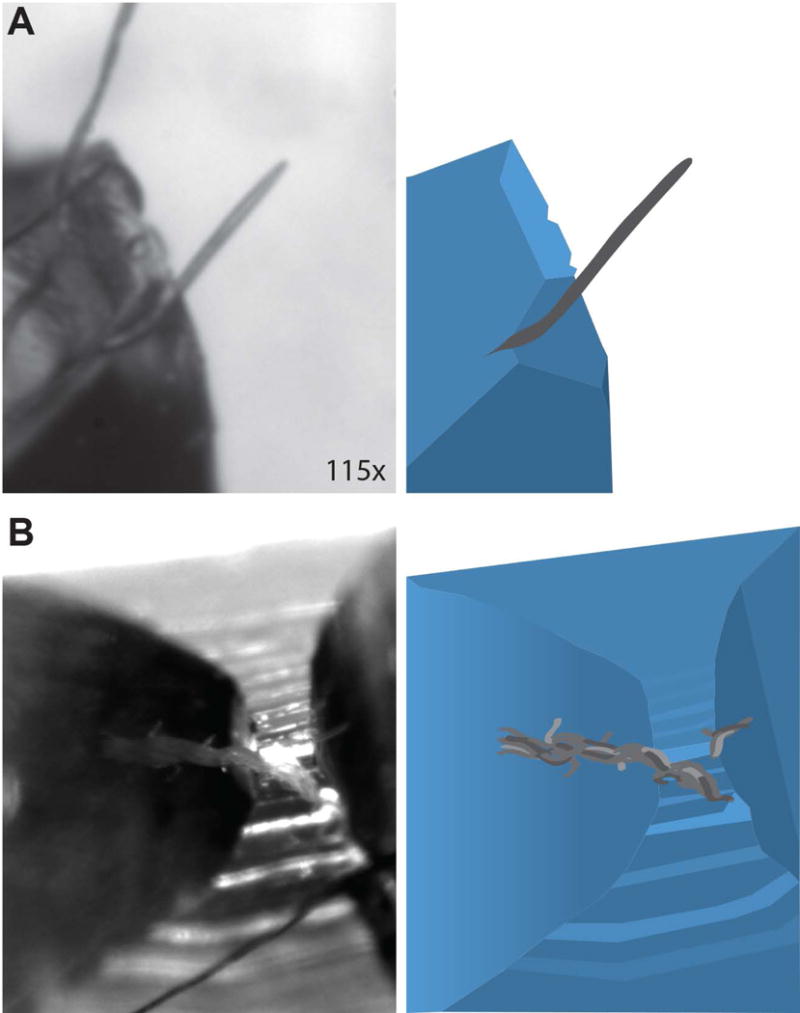
Micrographs (left) and cartoon schematics (right) of an individual nictating dauer (A) and a swarm of elevated dauers (B) on top of a PDMS pedestal. Individual dauers are capable of standing on their tail while remaining in a rigid posture for several seconds. Swarms consist of tens of dauers undulating in the air together, essentially using the neighboring animal as a structure to climb. The base of the swarm is indicated with a red arrow while the top is indicated with a yellow arrow.
Box 1. Was swarming by dauers the first recorded N2 phenotype?
The most commonly used C. elegans laboratory strain is the Bristol N2 strain. N2 was given to Sydney Brenner in 1964 by Ellsworth Dougherty who received it from Warwick Nicholas.108 Nicholas received N2 from Lancelot Staniland, a nematologist with the National Agricultural Advisory Service in Bristol, U.K., who originally isolated N2 from mushroom compost.109 In addition to several books on art technique, Staniland’s research focused primarily on foliar plant-parasitic nematodes.110–113 In 1957 Staniland described a swarming behavior of Rhabditid dauer “eelworms” in mushroom houses.114 Staniland could disrupt dauer swarming behavior by touch and could induce swarming behavior by exposure to light. Interestingly, C. elegans is photoresponsive despite the absence of eyes or ocelli.115 Finally, Staniland noted a general lack of males, a trait found in C. elegans and a subset of other Caenorhabditis species.116 While it is tempting to speculate that N2 was among the nematodes described in this report, Staniland did not identify the nematodes in this population to the species level. Furthermore, the nematodes in his report were isolated from near Dorset, approximately 100 km south of Bristol.
The dauer stage is an ecologically relevant component of many nematode species. Recent years have seen an increased interest in the natural history of C. elegans. Although widely distributed across multiple continents, undisturbed soils rarely contain C. elegans.17 C. elegans is most commonly found in the dauer stage in rotting vegetable matter or associated with arthropods.18 While Cassada and Russell contrasted dauers with “normal” (i.e. non-dauer) larva, 15 we believe the evidence from recent ecological studies demonstrates that there is nothing abnormal regarding the dauer state. In fact, it is likely that the dauer stage serves as a primary survival mechanism during the bust phase of the C. elegans boom-bust life history strategy.17 Several parasitic nematodes form dauers that serve as the infective stage during infection. The dauer stage of C. elegans is considered homologous to the infective stage of many parasitic nematodes and is used a comparator to understand the developmental biology of several devastating pathogens (See Box 2).19
Box 2. The dauer stage is homologous to the infective stage of many parasitic nematodes.
Nematodes are among the most devastating parasites of plants and animals. Many researchers have commented on the similarity between the dauer stage of C. elegans and the infective stage of parasitic nematodes leading to a hypothesis that dauers in free-living (non-parasitic) nematodes are an evolutionary stepping stone to parasitism.19 Similar to dauers, the infective stage of many parasitic nematodes are non-feeding and morphologically distinct from other developmental stages. Frequently, the infective stage of parasitic nematodes also displays stage-specific behaviors, including nictation, that facilitate infection of the host.117 Several groups have used the similarity between dauers and infective-stage parasites to explore mechanisms of development in parasitic species. For example, Gerry Schad and colleagues combined transmission electron microscopy (TEM) and laser ablation to demonstrate that neurons homologous to those controlling C. elegans dauer entry, regulate entry into the infective stage in the parasite Strongyloides stercoralis.118 Similarly, ablation of the ASJ sensory neuron, which regulates dauer recovery in C. elegans, also regulates recovery from the infective stage in both S. stercoralis and Heterorhabditis bacteriophora.75,118,119 Molecular pathways regulating dauer formation and infective stage developmental dynamics appear conserved between C. elegans and many parasitic species.19,120,121
THE DAUER FORMATION DECISION
Several excellent reviews discuss the primary environmental components and genetic pathways regulating the decision to enter dauer. 20–22 We will give only a brief overview of the mechanisms involved in the decision to enter dauer (Figure 3). Dauer formation is regulated by three environmental components: food, a pheromone indicating population density, and temperature.13,23,24 Low levels of food promote dauer formation whereas plentiful food inhibits dauer formation. Conversely, high levels of pheromone promote dauer formation while low levels of pheromone prevent dauer formation. In addition to food and pheromone, temperature serves a modulatory role such that higher temperatures, within the normal physiological range (15-25°C), promote dauer formation.13 Temperatures above this range can result in constitutive dauer formation in genetic backgrounds not typically associated with a dauer constitutive phenotype.25
Figure 3. The dauer formation decision pathway.
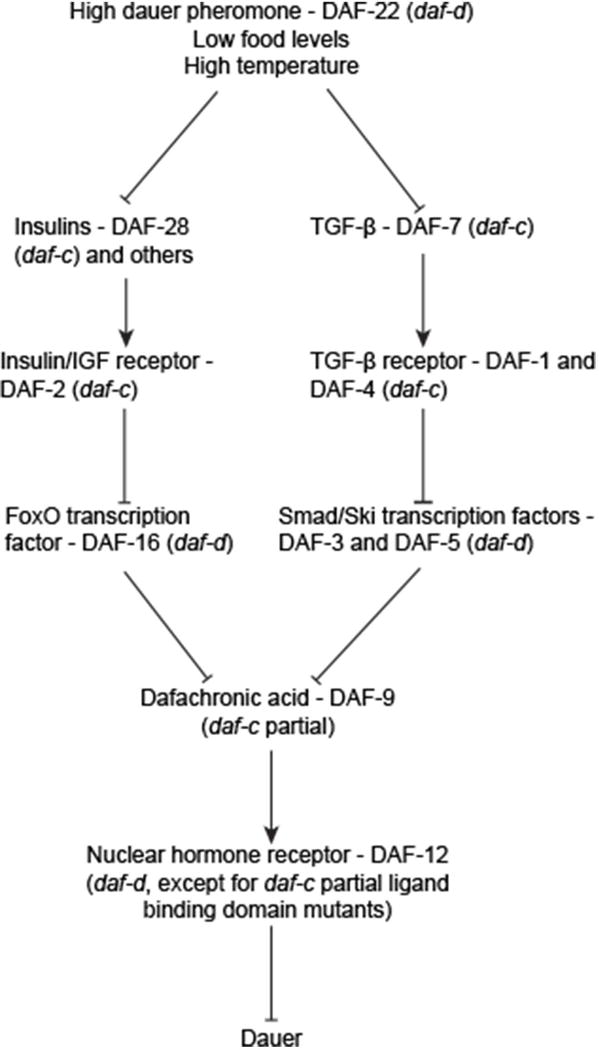
The decision to enter dauer begins with environmental components and proceeds through two parallel pathways before converging onto a lipid hormone signalling pathway. Mutating the encoding genes of this pathway may lead to either constitutive dauer formation (daf-c, dauer formation constitutive) or an inability to form dauers (daf-d, dauer formation defective). Lines ending with arrows indicate increased activity of the downstream component. Lines ending with bars indicate suppression or downregulation of the downstream component.
Both forward and reverse genetics approaches combined with detailed epistasis analysis have elucidated the genetic pathways required for the decision to enter or bypass dauer. Mutagenesis screens have uncovered several genes that when perturbed, result in altered dauer formation. Mutants from these screens are grouped into daf-c (dauer formation abnormal constitutive) mutants that form dauers in favorable environmental conditions and daf-d (dauer formation abnormal defective) mutants that cannot form dauers even under dauer-inducing conditions.
Chemosensory neurons in the head of C. elegans sense the presence of the pheromone.26 In plentiful food and low-population density, sensory neurons release insulins and TGF-β and thereby activate two parallel pathways (Figure 3).27,28,29 The insulin signalling pathway is characterized by the insulin receptor ortholog DAF-2, which inhibits the downstream Forkhead transcription factor DAF-16. 30,31,32 DAF-7/TGF-β is recognized by the cognate receptors DAF-1 and DAF-4, which then inhibit the DAF-5 and DAF-3 transcription factors.27,33 Interestingly, these pathways appear conserved in the development of infective stages of some parasitic nematodes (See Box 2). The insulin and TGF-β parallel pathways converge onto the cytochrome P450 enzyme, DAF-9.34,35,36,37 In favorable environmental conditions, DAF-9 acts to produce lipid-based hormones called dafachronic acids.38 The dafachronic acids bind to the DAF-12 nuclear hormone receptor to promote non-dauer development. While daf-12 null mutants are daf-d, unbound DAF-12 is required for dauer formation. The result is that specific mutations in the ligand-binding domain encoding region of daf-12 result in constitutive dauer formation. 39
WHAT IS A DAUER?
Based on our knowledge regarding dauer formation, this question seems deceptively simple. Cassada and Russell defined the C. elegans dauer as both “a morphologically recognizable, nongrowing stage” and “a juvenile stage specialized for survival under harsh conditions”.15 The first definition focuses on morphology and development while the second relies primarily on function. Since that time, extensive analysis of gene expression changes during dauer has contributed to our understanding of dauer-specific characteristics.40,41 As discussed previously, the most common test for “dauerness” relies on survival to SDS. However, a single functional determination of dauer may overlook important tissue remodeling events that contribute to aspects of the dauer stage. A recent alternative non-destructive assay uses the ingestion of fluorescent beads to distinguish the non-feeding dauers from non-dauers.42 However, these tests do not depict all of the structural changes that occur during dauer.
We, therefore, review the individual remodeling events that occur during dauer and, where available, provide molecular mechanisms regulating changes to particular tissues. In addition to genes that control single dauer remodeling phenotypes, there are several pathways that, when disrupted, lead to pleiotropic defects in dauer morphology. We discuss these “partial dauer” pathways in the subsequent section.
Changes to the cuticle and underlying epidermis
Nematodes are wrapped in a multilayered collagen-based cuticle.43 Dauers show large differences in cuticular structure compared with non-dauers. In cross-section, the outer layer of the dauer cuticle is thicker than in non-dauers. Dauers also contain an additional striated layer that is not found in non-dauers.15 Furthermore, biochemical differences distinguish dauer from non-dauer cuticles.44–46 These differences likely mediate resistance to environmental stressors. For example, disruption of the epithelial-expressed sugar transporter SRF-3 leads to altered cuticular antigenicity and sensitivity to SDS during dauer.47,48
The most visible structural change to the cuticle during dauer is the formation of longitudinal ridges of raised cuticle called ‘alae’ running along the length of the lateral sides (Figure 4A). The function of the lateral alae is unknown although they are speculated to aid in nematode movement.49,50 The production of the alae appears directly related to the radial shrinkage that occurs during dauer formation. The lateral alae are produced by a series of underlying epithelial stem-cell like ‘seam’ cells that shrink during dauer (Figure 4B).51 Ablation of individual seam cells leads to gaps in alae formation and corresponding areas lacking radial shrinkage.51 Shrinkage of the seam cells during dauer is regulated through autophagy (see Partial Dauer section).52 In addition to collagen, nematode cuticles contain a collagenase insoluble fragment termed ‘cuticulins’.53 Proper dauer alae formation requires the zona pellucida domain-containing cuticulins CUT-1, CUT-5, and CUT-6.54,55
Figure 4. Seam cell shrinkage and cuticle remodeling occur during dauer formation.
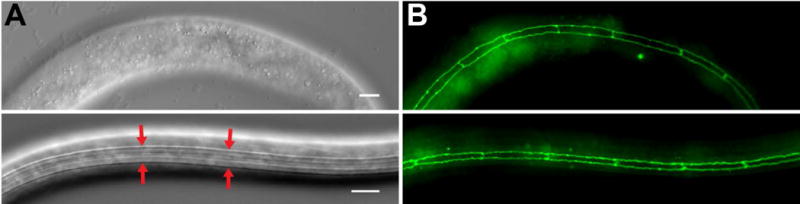
DIC (A) and fluorescent (B) images of non-dauer L3 (top) and dauer (bottom) ajm-1::gfp larvae. During dauer the lateral cuticle contains raised ridges called alae (red arrows). Production of alae is correlated with shrinkage of the underlying epithelial seam cells as seen with the ajm-1::gfp transgene. Scale bars,10 μm.
Several ‘heterochronic genes’ are also needed for proper alae formation. Heterochronic mutants have defects in the timing of developmental events including dauer formation.56,57 For example, disruption of the LIN-14 transcription factor results in precocious dauers that form following the molt into L2.57 These lin-14 loss of function precocious dauers form typical dauer lateral alae. In contrast, a heterozygous gain of function lin-14 mutant may form SDS-resistant dauer following the molt into L3 and stronger gain of function alleles block dauer formation altogether. Among the various heterochronic genes, some mutants form incomplete dauers at the appropriate developmental stage.57 For example, lin-28 mutants form mosaic dauers with patches of both dauer-specific alae and non-dauer cuticle. Interestingly, passage through the dauer stage can suppress other mutant non-dauer phenotypes found in some heterochronic mutant animals.58–63 Contrary to the traditional view that passage through the dauer stage does not impact post-dauer fitness in wild-type animals,14 more recent work demonstrated that post-dauer adults have distinct gene expression profiles and increased longevity and fecundity compared with adults that bypassed the dauer stage.64
Another striking anatomical change during dauer is the formation of a cuticle-based buccal plug which seals the mouth (Figure 6A).65 The cellular source of the buccal plug is unknown. Some partial dauer mutants (see below) have defects in buccal plug formation. In addition to the lateral seam cells, much of the epithelium comprises a set of hypodermal cells and syncytia. Increased lipid storage occurs within the hypodermis during dauer formation.66 Several of the partial dauer pathways regulate the increased lipid storage found in dauers.
Figure 6. The non-feeding dauer stage is characterized by buccal cavity occlusion and pharyngeal bulb shrinkage.

DIC micrographs of L3 (top) and dauer (bottom) animals demonstrating changes to the buccal cavity (A, red arrow) and the pharynx (B). Shrinkage of the pharynx is most obvious in the terminal bulb (red arrow). Scale bars, 10 μm
Changes to muscle
Neuromuscular junctions in C. elegans form between muscle arms, which extend from the contractile portion of the muscle, and motor neurons.67 Dauers have more muscle arms compared to non-dauers.68 Unlike many other dauer-specific changes to morphology, these additional muscle arms persist in post-dauer adults.68 Interestingly the daf-2 insulin signaling mutant (Figure 3) shows an increased number of muscle arms during non-dauer stages.67 While the number of muscle arms increases during dauer, it is not known if the density of neuromuscular junctions also changes during dauer. Dauer muscles also display changes in mitochondrial ultrastructure.65 One possible function for muscle remodeling is the promotion of dauer-specific behaviors such as nictation. During movement on a flat, two-dimensional surface, C. elegans moves through a coordinated contraction and relaxation of body wall muscles to produce a sinusoidal movement. The standing behavior seen during dauer nictation (Figure 2) may require coordination of muscles to allow for simultaneous contraction.
Changes to the nervous system
Widespread and dramatic morphological changes occur throughout the nervous system during dauer that may mediate behavioral changes. Some changes to the nervous system are reversible upon exit from dauer while others are retained following dauer recovery. The amphids are the primary sensory organs in C. elegans comprising 12 pairs of ciliated sensory neurons and two pairs of glial cells that wrap around the ending of amphid neurons.69 A subset of amphid neurons and glia remodel as the animal enters dauer.70
The AFD amphid neurons are temperature sensing neurons containing finger-like microvilli at the distal tip.69,71 Dauers contain approximately twice as many AFD microvilli as non-dauers.70 Interestingly, a mixture of starved non-dauers and dauers showed an opposite response to thermal gradients as well-fed non-dauers.72 However, it is not known if ultrastructural changes to AFD mediate changes in thermotaxis behavior. Many AFD microvilli are lost upon recovery from dauer.70
The AWC amphid neurons sense volatile odorants and extend ciliated sheets (wings) dorsally and ventrally at the distal end of the dendrite.69 In non-dauers, the wing structure of the left and right AWC extend out in a slight arc of approximately 100° around the circumference of the nose. During dauer, the wings increase in overall size resulting in a greater degree arc (240°) and overlapping dorsal and ventral wings from each AWC neuron.70,73 The function of AWC remodeling during dauer is not known. It will be interesting to explore the cytoskeletal changes that occur during dauer remodeling of the morphologically distinct AWC cilia.
The endings of the two amphid sheath glia cells also expand. The amphid sheath glia wrap around the end of the amphid neuron bundle and envelop the wing component of AWC in non-dauers.69 During dauer the sheath glia endings expand along with AWC; the left and right sheath endings of the sheath glia fuse in approximately half of dauers.70,73 Ablation of the sheath glia cell during L1 results in a complete absence of the AWC wing structure and loss of AWC chemosensory function in non-dauers as well as a failure to expand AWC wings during dauer, suggesting that the glia are required for proper neuron remodeling.74 Conversely, ablation of the AWC neuron does not affect sheath remodeling.73 These results suggest that AWC remodeling is dependent on sheath remodeling, but not vice versa. The cell fusion gene aff-1 is required for sheath glia fusion during dauer. Similarly, the homeodomain transcription factor TTX-1 and glial-specific transciption factor ZTF-16 regulate the receptor tyrosine kinase VER-1 to promote sheath glia fusion during dauer.73,75 Unlike other neuronal remodeling events during dauer, the changes to AWC and the sheath cells persist following dauer recovery.70,73
ASG and ASI are amphid neurons with single cilia projecting into the amphid pore.69 Both ASG and ASI regulate dauer formation.29,76 During dauer, the cilia of these neurons are posteriorly displaced by 2-3 μm compared to non-dauers.70 In ASI, this displacement translates to incompletely penetrant defects in staining by a lipophilic fluorescent dye.77 It would be interesting to determine whether the partial defect in ASI dye-filling correlates with the physiological age of dauers or variability in dauer-specific behaviors. Remodeling of ASG and ASI is reversible upon recovery from dauer.70
Separate from the amphids, the six inner-labial sensilla each contain two ciliated neurons (IL1 and IL2). During dauer, the IL2s undergo extensive structural remodeling. Electron micrographs demonstrate that the IL2 cilia are approximately two-thirds shorter during dauer than non-dauer and no longer extend through the opening of the inner-labial pore.70 Similar to ASI, the structural changes in IL2 cilia may account for the lack of IL2 dye-filling during dauer.78 In addition to the shortening of the cilia, the four dorsal and ventral IL2s (quadrant IL2s) undergo extensive dendrite arborization during dauer formation leading to a three-fold increase in total dendritic length (Figure 5).79 The two lateral IL2 neurons branch only once at the distal end of the dendrite during dauer. The remodeling of the IL2 neurons is reversible. Following recovery from dauer, the IL2 arbor resorbs through unknown mechanisms leaving only occasional remnant branches.79 IL2 arborization is regulated by the proprotein convertase KPC-1 and the POU homeodomain transcription factor UNC-86.79 Interestingly, arborization of the PVD and FLP neurons also require kpc-1 and unc-86.79,80 However, PVD and FLP do not arborize during dauer (unpublished observation) suggesting distinct mechanisms regulate stress-induced (IL2) neuron branching. The IL2s are required for dauer-specific nictation behavior.16 While IL2-specific rescue of kpc-1 mutants rescued nictation defects, how the IL2 arbor regulates nictation remains to be determined.79
Figure 5. IL2 dendrites arborize during dauer.
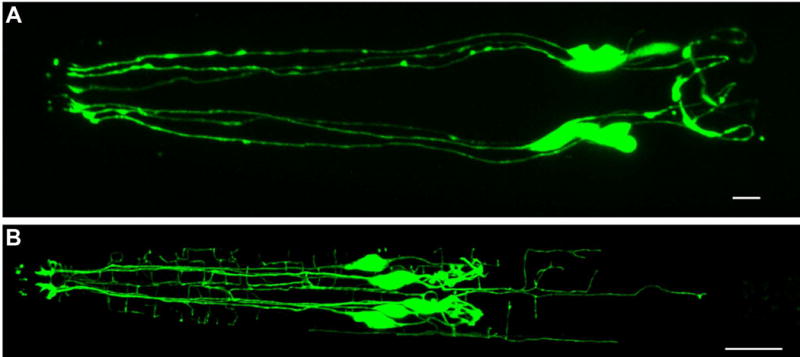
Confocal micrographs of an L3 (A) and dauer (B) animal expressing klp-6p::gfp in the six IL2 neurons. During non-dauer stages the IL2s are unbranched with a single dendrite projecting anteriorly (left) and a single axon projecting posteriorly (right) from the cell body. During dauer the IL2s arborize extensively, increasing their total dendritic length three-fold, with the bulk of their arbors originating from just four of the six cells. Scale bars, 10 μm.
The paired ciliated sensory deirid neurons (ADE) are located dorsolaterally approximately 80μm posterior of the nose.69 In non-dauers, the ciliated tips of ADE are embedded in the cuticle and function in mechanosensation.69,81,82 In dauers, the cilia are reoriented and held in place by a dauer-specific substructure.70 The substructure is visible with light microscopy and produced by the deirid socket glia cell.83 Neither the genetic mechanisms regulating ADE remodeling nor their function during dauer is known.
Changes to the pharynx and gut
During dauer the pharynx shrinks (Figure 6B) and pharyngeal pumping, which allows for ingestion of bacteria, is suppressed. Despite the change in morphology, the dauer pharynx is capable of pumping 84; however the buccal plug (See Changes to cuticle) prevents entry of any food (Figure 6A). Several ‘partial dauer’ genes regulate remodeling of the pharynx (See below). Posterior of the pharynx, the intestinal lumen is shrunken with small and indistinct microvilli. The dauer intestine contains electron dense ‘cytosomes’.65 While the remodeling of the intestine correlates with a lack of ingestion, it is not clear whether the shrinking of the microvilli is due to a lack of feeding or is a more specific response of dauer remodeling. It has also been suggested that the dauer anus is occluded;85,86 however, recent data from our lab (unpublished) and scanning electron micrographs indicate that the anus is not blocked during the dauer stage.87
Changes to the gonad
While the gonad does not show apparent remodeling during dauer, the proliferation of the germline and somatic gonad halts. During dauer the gonad arrests at a stage consisting of twelve somatic cells and approximately 40 germ cell nuclei.88,89 Germline arrest successfully preserves the viability of the germ cells. In fact, development through dauer results in slightly increased lifespan and brood size in post-dauer adults compared with animals that bypass the dauer stage.64
Changes to the excretory system
The excretory system of C. elegans consists of four cell types (excretory, duct, pore and gland) and, unlike the occlusion of the buccal cavity, remains open to the environment during dauer. The excretory system regulates osmotic pressure. Ablation of the excretory, duct or pore cells causes animals to fill with fluid and die within a day.90 During dauer, the gland cell lacks the secretory granules typically found in non-dauers.91 However, ablation of the gland cells has no apparent effect on dauer formation or recovery.90 During dauer, the duct cell visibly pulsates. Although highly variable, the pulsation negatively correlates with the osmotic pressure of the surrounding medium. Exposing dauers to distilled water increases the pulsation compared to dauers in M9 buffer.90 The pulsation may be due to an increased requirement for osmotic regulation during dauer arising from the obstruction of the buccal cavity or changes to the cuticle.90 There are no observable neuromuscular inputs to the duct cell and the molecular basis of duct cell pulsation during dauer is unknown.91
PARTIAL DAUERS, DAUER-LIKE LARVAE, AND DAUER MOSAICS: MUTANTS WITH PLEIOTROPIC DEFECTS IN DAUER REMODELING
Dauer formation is considered a binary developmental decision.92 However, many mutants produce dauers with defects in several morphological characteristics. While the literature gives different names for this class of phenotype, we will use the term partial dauer. There is no single partial dauer phenotype; we define partial dauers as those that arrest after L2d but are missing or are incomplete for more than one dauer morphological feature. While we discuss those pathways with known pleiotropic effects in dauer remodeling, it is possible that mutants previously described to have only single phenotypic effects during dauer remodeling produce subtle changes to various dauer tissues.
Examination of partial dauers may elucidate our understanding of dauer remodeling. However, two confounding factors make a comprehensive post hoc analysis of partial dauers challenging. First, quantitative data of partial dauer morphology is usually lacking. For example, radial and pharyngeal shrinkage are often classified either as dauer-like, non-dauer or as intermediate. Second, with few exceptions, it is unclear whether a partial dauer represents incomplete remodeling during dauer formation or a stalled recovery transition. Despite these challenges, the phenotypic variability among partial dauers provides a potentially rich data source for understanding phenotypic plasticity. Below, we describe several well-known molecular pathways that result in partial dauers when disrupted. While listed separately, many of these pathways interact. For example, daf-16 is upstream of the daf-9 lipid hormone signaling pathway. 36,37,93
daf-9, daf-12, and lipid hormone signaling
The term “dauer-like larvae” was coined by Don Riddle and Patrice Albert regarding the phenotypes of daf-9 and daf-15.93 As mentioned earlier, daf-9 is required for synthesis of dafachronic acid. Production of dafachronic acid is associated with plentiful environmental conditions and results in bypassing of the dauer stage. As expected, daf-9 mutants are dauer constitutive. However, unlike other daf-c mutants located earlier in the dauer formation pathway (Figure 3), daf-9 null mutants form only partial dauers with variable defects in dauer remodeling.92 While daf-9 partial dauers have alae, the radial shrinkage is intermediate between a true dauer and an L3.93 Similarly, while the buccal cavity is occluded in daf-9 mutants, there are ultrastructural differences in the lining of the cavity between the daf-9 partial dauer and wild-type dauers. daf-9 mutants display sporadic pharyngeal pumping and are intermediate in their survival to SDS.93
The DAF-12 nuclear hormone receptor for dafachronic acids is essential for dauer formation. daf-12 null alleles are completely dauer defective and epistatic to all daf-c mutations, confirming its role at the end of the dauer decision pathway. However, there is substantial diversity in the phenotype among daf-12 mutant alleles.39,94 The DAF-12 nuclear hormone receptor contains both DNA and ligand-binding domains.94 Contrary to the null alleles, mutations in the ligand-binding domain lead to a daf-c phenotype.94 Similar to the daf-9 mutants, disruption of the daf-12 ligand-binding domain leads to a partial dauer phenotype.39
Several additional genes that act in the dafachronic acid pathway lead to partial dauer formation. The tyrosine phosphatase SDF-9 (Synthetic Dauer Formation) assists in the function of DAF-9. Similar to the daf-9 mutations, sdf-9 mutants form partial dauers that exhibit non-dauer pharyngeal morphology.95 Similarly, the Niemann-Pick disease protein homologs NCR-1 and NCR-2 act upstream of DAF-9 for dafachronic acid production and ncr-1; ncr-2 double mutants mimic the partial dauer phenotype of daf-9 mutants.96
Dafachronic acid is synthesized from a cholesterol precursor, but C. elegans is not capable of manufacturing its own cholesterol.97 In the laboratory, cholesterol is added to C. elegans culture media.6 Interestingly, weak alleles of daf-9, as well as sdf-9 and ncr-1;ncr-2 mutants show an increased penetrance of partial dauer formation when grown in low-cholesterol conditions.36,37,95,96 Growth of wild-type C. elegans on media completely deficient in cholesterol will also result in partial dauers that mimic the daf-9 mutant phenotype.36 Interestingly, replacement of cholesterol with lophenol, a methylated cholesterol derivative, induces complete dauer formation in wild-type C. elegans under non-dauer inducing conditions.98
daf-16
Mutations in the Forkhead Box O (FOXO) transcription factor-encoding gene, daf-16 are daf-d and suppress the constitutive dauer formation in daf-2 mutants suggesting that daf-16 is epistatic to daf-2. In addition to its role in dauer formation, the DAF-2/DAF-16 insulin signaling pathway has gained significant attention as a primary regulator of lifespan. While daf-16 mutants are short-lived, daf-2 mutants are very long-lived.99 Growth of non-null daf-16 single mutants on high levels of dauer pheromone and low levels of food can induce a transient dauer state with SDS resistance, but without a remodeled pharynx.100 The penetrance of this transient dauer state is the result of an interaction effect between the mutant allele and the environment; daf-16(m26) mutants form a higher percentage of transient dauers in liquid culture while daf-16(m27) dauers form a higher percentage on agar media.100,101 The mechanism behind this allele-environment interaction is unknown.
While daf-16 mutations completely suppress the constitutive dauer formation of daf-2 mutants, daf-16; daf-7 double mutants form almost 100% partial dauers at 25°C.31,100,101 daf-16; daf-7 partial dauers show dauer-specific alae although they frequently appear less distinct than in complete dauers. While the darkness of the daf-16; daf-7 intestine is intermediate between dauers and non-dauers 101, there was no reduction in fat accumulation compared to true dauers.100 The pharynx of daf-16, however, appears closer in morphology to non-dauers and will frequently pump.101 daf-16 is downstream of daf-2 in a separate pathway from DAF-7 TGF-β signaling. Interestingly, double mutants such as daf-2; daf-3 that also combine daf-c and daf-d mutants from the insulin signaling and TGF-β pathways (Figure 3) do not result in a partial dauer phenotype.101 One interpretation of these data is that DAF-16 functions during both the dauer formation decision and dauer morphogenesis. This possibility is supported by the fact that growth of daf-16 mutants on lophenol results in partial dauers whereas wild-type animals form complete dauers when grown on lophenol.98
mTOR and autophagy
Similar to daf-9 mutants, daf-15 mutants were described as dauer-like larvae that either never exit dauer or exit only to become sterile adults.93 daf-15 encodes a homolog to RAPTOR the (Regulatory-associated Protein of mTOR). Unlike daf-9 mutants, disruption of daf-15 leads to an arrested stage with very few recognizable dauer characteristics.93 While daf-15 mutants arrest at the second molt, they do not show any alae, radial constriction, pharyngeal remodeling, or buccal plug formation and lack resistance to SDS.93 However, daf-15 mutants do show a dark intestine typical of dauers and neuroanatomy that is intermediate between dauers and non-dauers.93 Mutants in the mTOR homolog let-363 produce a similar partial dauer phenotype as daf-15.102
mTOR is a major regulator of autophagy, a conserved cellular response to starvation and other stressors. In a daf-2 daf-c mutant background, RNAi of conserved autophagy genes leads to the formation of partial dauers with partial radial shrinkage, incomplete pharyngeal remodeling and a lack of SDS resistance.52 Expression of the canonical marker of autophagy, LGG-1, is upregulated in dauer. Ultrastructural examination of bec-1 RNAi treated daf-2 mutant animals demonstrated that autophagy was necessary for the shrinkage of seam cells during dauer formation.52
AMPK
The 5′ adenosine monophosphate-activated protein kinase (AMPK) subunits AAK-1 and AAK-2 play multiple roles in dauer remodeling. Disruption of the AMPK subunits leads to a loss of germline quiescence during dauer.89 While aak-2 mutants are resistant to SDS, they display incomplete remodeling of the pharynx and radial shrinkage of the body.88 AAK-2 was also implicated in maintenance of lipid stores during dauer 103; however, recent work has suggested that the effect of aak-2 on lipid stores is not dauer-specific.104
CONCLUSIONS
C. elegans dauers exhibit large-scale remodeling of most tissues. However, compared with the adult hermaphrodite, there is a dearth of ultrastructural data on dauer. It is likely that additional remodeling events during dauer will be uncovered. For example, are the downstream circuits of the IL2s similar to those found in non-dauers? Does the increased number of muscle arms correspond to changes in the number or distribution of neuromuscular junctions? A more detailed examination of anatomy during dauer may lead to the discovery of new remodeling phenotypes. Because the dauer stage is ultimately a response to the environment, it may be interesting to examine the variability in dauer remodeling under slightly varying environmental conditions. It seems unlikely that all dauers are equal. For example, we observe more variability among dauers in the branching pattern of the IL2s than seen among non-dauers in the highly branched PVD neurons (unpublished). Golden and Riddle noted that older dauers were more liable to recover than young dauers under equivalent environmental conditions.13
While the pathways regulating the dauer entry decision are well studied, less attention has been given to molecular mechanisms regulating how individual tissues remodel. Both dauer-specific and shared (i.e. between dauer and non-dauer development) gene expression regulate remodeling. Given the prevalence of dauers from isolations in nature 17, it is logical that part of the genome is devoted to dauer-specific remodeling. For example, the cuticulin gene cut-1 is expressed exclusively in dauers and required for proper dauer alae formation.54 Genetic screens will likely identify new remodeling genes that may provide insight into stress-induced remodeling during normal mammalian development and mechanisms of stress-associated pathologies.
A careful analysis of partial dauers presents an opportunity to dissect the mechanisms coordinating remodeling. For example, several of the partial dauer mutants have both incomplete radial shrinkage of the body and the pharynx. Does the mechanical shrinkage of the body induce a similar contraction in the pharynx? A quantitative analysis of this and other aspects of dauer remodeling in several partial dauer mutants, followed by multivariate statistical analysis may elucidate whether different tissues coordinate during dauer. However, it will be important to differentiate between partial remodeling during dauer formation and a stalled stage of recovery. Differentiating between these possibilities should be possible by producing a detailed timeline of all remodeling events during dauer formation and then using highly synchronized populations for assays. Extending the question of partial dauer formation vs. stalled recovery; is proper remodeling required for maintaining the dauer state? If so, it is likely we may miss certain genes regulating dauer remodeling.
Is it possible that partial dauers exist in nature? Ailion and Thomas speculated that partial dauers may form in a wild-type background under certain environmental conditions that would favor specific remodeling events.105 Indeed, wild-type animals that are deprived of cholesterol will form partial dauers that phenocopy the daf-9 mutants.36 As C. elegans is found in diverse climates and environments 17, it seems likely that environmental conditions may occasionally favor partial remodeling. West-Eberhard proposed that changes due to phenotypic plasticity may become fixed during evolution and thereafter lead to speciation.1,106 There is substantial variation in the dauer formation decision among diverse C. elegans isolates.107 It may be informative to examine the diversity of wild Caenorhabditis isolates for variation in dauer morphology and behavior.
Acknowledgments
We thank Roland Perry and David Hooper for providing information about Lancelot Staniland and two anonymous reviewers for providing valuable suggestions. We are funded by the NIH 1R01GM111566.
Contributor Information
Rebecca J. Androwski, Neuroscience Program, University of Illinois at Urbana-Champaign
Kristen M. Flatt, Neuroscience Program, University of Illinois at Urbana-Champaign
Nathan E. Schroeder, Neuroscience Program and Dept. of Crop Sciences, University of Illinois at Urbana-Champaign
References
- 1.West-Eberhard MJ. Developmental plasticity and evolution. New York: Oxford University Press; 2003. [Google Scholar]
- 2.McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. https://doi.org/10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pener MP, Simpson SJ. Locust phase polyphenism: an update. Adv In Insect Phys. 2009;36:1–272. https://doi.org/10.1016/S0065-2806(08)36001-9. [Google Scholar]
- 4.Shuel RW, Dixon SE. The early establishment of dimorphism in the female honeybee, Apis mellifera L. Insectes Soc. 1960;7:265–282. https://doi.org/10.1007/BF02224497. [Google Scholar]
- 5.Mao W, Schuler MA, Berenbaum MR. A dietary phytochemical alters caste-associated gene expression in honey bees. Sci Adv. 2015;1 doi: 10.1126/sciadv.1500795. https://doi.org/10.1126/sciadv.1500795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. https://doi.org/10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 8.Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. https://doi.org/10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- 9.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil Trans R Soc Lond B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 10.Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. https://doi.org/10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 12.Dickinson DJ, Goldstein B. CRISPR-based methods for Caenorhabditis elegans genome engineering. Genetics. 2016;202:885–901. doi: 10.1534/genetics.115.182162. https://doi.org/10.1534/genetics.115.182162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golden JW, Riddle DL. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev Biol. 1984;102:368–378. doi: 10.1016/0012-1606(84)90201-x. https://doi.org/10.1016/0012-1606(84)90201-X. [DOI] [PubMed] [Google Scholar]
- 14.Klass M, Hirsh D. Non-ageing developmental variant of Caenorhabditis elegans. Nature. 1976;260:523–525. doi: 10.1038/260523a0. [DOI] [PubMed] [Google Scholar]
- 15.Cassada RC, Russell RL. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. https://doi.org/10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- 16.Lee H, Choi M, Lee D, Kim H, Hwang H, Kim H, Park S, Paik Y, Lee J. Nictation, a dispersal behavior of the nematode Caenorhabditis elegans, is regulated by IL2 neurons. Nat Neurosci. 2012;15:107–112. doi: 10.1038/nn.2975. https://doi.org/10.1038/nn.2975. [DOI] [PubMed] [Google Scholar]
- 17.Frézal L, Félix M-A. C. elegans outside the Petri dish. Elife. 2015;4:e05849. doi: 10.7554/eLife.05849. http://dx.doi.org/10.1016/j.cub.2010.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Félix M-A, Braendle C. The natural history of Caenorhabditis elegans. Curr Biol. 2010;20:R965–R969. doi: 10.1016/j.cub.2010.09.050. https://doi.org/10.1016/j.cub.2010.09.050. [DOI] [PubMed] [Google Scholar]
- 19.Crook M. The dauer hypothesis and the evolution of parasitism: 20 years on and still going strong. Int J Parasitol. 2014;44:1–8. doi: 10.1016/j.ijpara.2013.08.004. https://doi.org/10.1016/j.ijpara.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fielenbach N, Antebi A. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 2008;22:2149–2165. doi: 10.1101/gad.1701508. https://doi.org/10.1101/gad.1701508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.PJ Hu. Dauer. Riddle DL, editor. WormBook. 2007:1–19. doi: 10.1895/wormbook.1.144.1. http://doi.org/10.1895/wormbook.1.144.1. [DOI] [PMC free article] [PubMed]
- 22.Riddle DL, Albert PS. Genetic and environmental regulation of dauer larva development. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C elegans II. 2nd. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1998. [PubMed] [Google Scholar]
- 23.Golden JW, Riddle DL. A Caenorhabditis elegans dauer-inducing pheromone and an antagonistic component of the food supply. J Chem Ecol. 1984;10:1265–1280. doi: 10.1007/BF00988553. https://doi.org/10.1007/BF00988553. [DOI] [PubMed] [Google Scholar]
- 24.Golden J, Riddle D. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science. 1982;218:578–580. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]
- 25.Ailion M, Thomas JH. Dauer formation induced by high temperatures in Caenorhabditis elegans. Genetics. 2000;156:1047–1067. doi: 10.1093/genetics/156.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vowels JJ, Thomas JH. Multiple chemosensory defects in daf-11 and daf-21 mutants of Caenorhabditis elegans. Genetics. 1994;138:303–316. doi: 10.1093/genetics/138.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren P, Lim C-S, Johnsen R, Albert PS, Pilgrim D, Riddle DL. Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. https://doi.org/10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Kennedy SG, Ruvkun G. daf-28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes Dev. 2003;17:844–858. doi: 10.1101/gad.1066503. https://doi.org/10.1101/gad.1066503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schackwitz WS, Inoue T, Thomas JH. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron. 1996;17:719–728. doi: 10.1016/s0896-6273(00)80203-2. https://doi.org/10.1016/S0896-6273(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 30.Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 31.Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 32.Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 33.Thomas JH, Birnby DA, Vowels JJ. Evidence for parallel processing of sensory information controlling dauer formation in Caenorhabditis elegans. Genetics. 1993;134:1105–1117. doi: 10.1093/genetics/134.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerisch B, Antebi A. Hormonal signals produced by DAF-9/cytochrome P450 regulate C. elegans dauer diapause in response to environmental cues. Development. 2004;131:1765–1776. doi: 10.1242/dev.01068. https://doi.org/10.1242/dev.01068. [DOI] [PubMed] [Google Scholar]
- 35.Mak HY, Ruvkun G. Intercellular signaling of reproductive development by the C. elegans DAF-9 cytochrome P450. Development. 2004;131:1777–1786. doi: 10.1242/dev.01069. [DOI] [PubMed] [Google Scholar]
- 36.Gerisch B, Weitzel C, Kober-Eisermann C, Rottiers V, Antebi A. A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Dev Cell. 2001;1:841–851. doi: 10.1016/s1534-5807(01)00085-5. https://doi.org/10.1016/S1534-5807(01)00085-5. [DOI] [PubMed] [Google Scholar]
- 37.Jia K, Albert PS, Riddle DL. DAF-9, a cytochrome P450 regulating C. elegans larval development and adult longevity. Development. 2002;129:221–231. doi: 10.1242/dev.129.1.221. [DOI] [PubMed] [Google Scholar]
- 38.Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, Suino-Powell K, Xu HE, Auchus RJ, Antebi A, et al. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 2006;124:1209–1223. doi: 10.1016/j.cell.2006.01.037. https://doi.org/10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 39.Antebi A, Yeh WH, Tait D, Hedgecock EM, Riddle DL. daf-12 encodes a nuclear receptor that regulates the dauer diapause and developmental age in C. elegans. Genes Dev. 2000;14:1512–1527. https://doi.org/10.1101/gad.14.12.1512. [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Kim SK. Global analysis of dauer gene expression in Caenorhabditis elegans. Development. 2003;130:1621–1634. doi: 10.1242/dev.00363. [DOI] [PubMed] [Google Scholar]
- 41.Jeong P-Y, Kwon M-S, Joo H-J, Paik Y-K. Molecular time-course and the metabolic basis of entry into dauer in Caenorhabditis elegans. PLoS One. 2009;4:e4162. doi: 10.1371/journal.pone.0004162. https://doi.org/10.1371/journal.pone.0004162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nika L, Gibson T, Konkus R, Karp X. Fluorescent beads are a versatile tool for staging Caenorhabditis elegans in different life histories. G3 Genes|Genomes|Genetics. 2016;g3(116):030163. doi: 10.1534/g3.116.030163. https://doi.org/10.1534/g3.116.030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bird AF, Bird J. The Structure of Nematodes. 2nd. San Diego: Academic Press; 1991. [Google Scholar]
- 44.Cox GN, Staprans S, Edgar RS. The cuticle of Caenorhabditis elegans. Dev Biol. 1981;86:456–470. doi: 10.1016/0012-1606(81)90204-9. https://doi.org/10.1016/0012-1606(81)90204-9. [DOI] [PubMed] [Google Scholar]
- 45.Blaxter ML. Cuticle surface proteins of wild type and mutant Caenorhabditis elegans. J Biol Chem. 1993;268:6600–6609. [PubMed] [Google Scholar]
- 46.Proudfoot L, Kusel JR, Smith HV, Harnett W, Worms MJ, Kennedy MW. Rapid changes in the surface of parasitic nematodes during transition from pre- to post-parasitic forms. Parasitology. 1993;107:107–117. doi: 10.1017/s0031182000079464. [DOI] [PubMed] [Google Scholar]
- 47.Link CD, Silverman MA, Breen M, Watt KE, Dames SA. Characterization of Caenorhabditis elegans lectin-binding mutants. Genetics. 1992;131:867–881. doi: 10.1093/genetics/131.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Höflich J, Berninsone P, Göbel C, Gravato-Nobre MJ, Libby BJ, Darby C, Politz SM, Hodgkin J, Hirschberg CB, Baumeister R. Loss of srf-3-encoded nucleotide sugar transporter activity in Caenorhabditis elegans alters surface antigenicity and prevents bacterial adherence. J Biol Chem. 2004;279:30440–30448. doi: 10.1074/jbc.M402429200. https://doi.org/10.1074/jbc.M402429200. [DOI] [PubMed] [Google Scholar]
- 49.Krall EL. In: Root Parasitic Nematodes Family Hoploaimidae. English, editor. New Delhi, India: Amerind Publishing; 1990. [Google Scholar]
- 50.Chitwood BG, Chitwood M. Introduction to Nematology. Consolidat, Baltimore, Maryland: University Park Press; 1974. [Google Scholar]
- 51.Singh RN, Sulston JE. Some observations on moulting in Caenorhabditis elegans. Nematologica. 1978;24:63–71. [Google Scholar]
- 52.Melendez A, Talloczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. https://doi.org/10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- 53.Fujimoto D, Kanaya S. Cuticlin: A noncollagen structural protein from Ascaris cuticle. Arch Biochem Biophys. 1973;157:1–6. doi: 10.1016/0003-9861(73)90382-2. https://doi.org/10.1016/0003-9861(73)90382-2. [DOI] [PubMed] [Google Scholar]
- 54.Sapio MR, Hilliard MA, Cermola M, Favre R, Bazzicalupo P. The Zona Pellucida domain containing proteins, CUT-1, CUT-3 and CUT-5, play essential roles in the development of the larval alae in Caenorhabditis elegans. Dev Biol. 2005;282:231–245. doi: 10.1016/j.ydbio.2005.03.011. https://doi.org/10.1016/j.ydbio.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 55.Muriel JM, Brannan M, Taylor K, Johnstone IL, Lithgow GJ, Tuckwell D. M142.2 (cut-6), a novel Caenorhabditis elegans matrix gene important for dauer body shape. Dev Biol. 2003;260:339–351. doi: 10.1016/s0012-1606(03)00237-9. https://doi.org/10.1016/S0012-1606(03)00237-9. [DOI] [PubMed] [Google Scholar]
- 56.Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226:409–416. doi: 10.1126/science.6494891. https://doi.org/10.1126/science.6494891. [DOI] [PubMed] [Google Scholar]
- 57.Liu Z, Ambros V. Heterochronic genes control the stage-specific initiation and expression of the dauer larva developmental program in Caenorhabditis elegans. Genes Dev. 1989;3:2039–2049. doi: 10.1101/gad.3.12b.2039. https://doi.org/10.1101/gad.3.12b.2039. [DOI] [PubMed] [Google Scholar]
- 58.Euling S, Ambros V. Reversal of cell fate determination in Caenorhabditis elegans vulval development. Development. 1996;122:2507–2515. doi: 10.1242/dev.122.8.2507. [DOI] [PubMed] [Google Scholar]
- 59.Liu Z, Ambros V. Alternative temporal control systems for hypodermal cell differentiation in Caenorhabditis elegans. Nature. 1991:162–165. doi: 10.1038/350162a0. [DOI] [PubMed] [Google Scholar]
- 60.Abrahante JE, Miller EA, Rougvie AE. Identification of heterochronic mutants in Caenorhabditis elegans: temporal misexpression of a collagen:: green fluorescent protein fusion gene. Genetics. 1998;149:1335–1351. doi: 10.1093/genetics/149.3.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abrahante JE, Daul AL, Li M, Volk ML, Tennessen JM, Miller EA, Rougvie AE. The Caenorhabditis elegans hunchback-like gene lin-57/hbl-1 controls developmental time and is regulated by microRNAs. Developmental cell. 2003;4:625–637. doi: 10.1016/s1534-5807(03)00127-8. [DOI] [PubMed] [Google Scholar]
- 62.Morita K, Han M. Multiple mechanisms are involved in regulating the expression of the developmental timing regulator lin–28 in Caenorhabditis elegans. The EMBO Journal. 2006;25:5794–5804. doi: 10.1038/sj.emboj.7601451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karp X, Ambros V. Dauer larva quiescence alters the circuitry of microRNA pathways regulating cell fate progression in C elegans. Development. 2012;139:2177–2186. doi: 10.1242/dev.075986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hall SE, Beverly M, Russ C, Nusbaum C, Sengupta P. A cellular memory of developmental history generates phenotypic diversity in C. elegans. Curr Biol. 2010;20:149–155. doi: 10.1016/j.cub.2009.11.035. https://doi.org/10.1016/j.cub.2009.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Popham JD, Webster JM. Aspects of the fine structure of the dauer larva of the nematode Caenorhabditis elegans. Can J Zool. 1979;57:794–800. doi: 10.1139/z78-217. https://doi.org/10.1139/z79-098. [DOI] [PubMed] [Google Scholar]
- 66.Hellerer T, Axäng C, Brackmann C, Hillertz P, Pilon M, Enejder A. Monitoring of lipid storage in Caenorhabditis elegans using coherent anti-Stokes Raman scattering (CARS) microscopy. Proc Natl Acad Sci U S A. 2007;104:14658–14663. doi: 10.1073/pnas.0703594104. https://doi.org/10.1073/pnas.0703594104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.White JG, Southgate E, Thomson JN, Brenner S. The structure of the ventral nerve of Caenorhabditis elegans. Phil Trans R Soc Lond B Biol Sci. 1976;275:327–348. doi: 10.1098/rstb.1976.0086. [DOI] [PubMed] [Google Scholar]
- 68.Dixon SJ, Alexander M, Chan KKM, Roy PJ. Insulin-like signaling negatively regulates muscle arm extension through DAF-12 in Caenorhabditis elegans. Dev Biol. 2008;318:153–161. doi: 10.1016/j.ydbio.2008.03.019. https://doi.org/10.1016/j.ydbio.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 69.Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J Comp Neurol. 1975;160:313–337. doi: 10.1002/cne.901600305. https://doi.org/10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- 70.Albert PS, Riddle DL. Developmental alterations in sensory neuroanatomy of the Caenorhabditis elegans dauer larva. J Comp Neurol. 1983;219:461–481. doi: 10.1002/cne.902190407. https://doi.org/10.1002/cne.902190407. [DOI] [PubMed] [Google Scholar]
- 71.Mori I, Ohshima Y. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature. 1995;376:344–348. doi: 10.1038/376344a0. https://doi.org/10.1038/376344a0. [DOI] [PubMed] [Google Scholar]
- 72.Hedgecock EM, Russell RL. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1975;72:4061–4065. doi: 10.1073/pnas.72.10.4061. https://doi.org/10.1073/pnas.72.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Procko C, Lu Y, Shaham S. Glia delimit shape changes of sensory neuron receptive endings in C. elegans. Development. 2011;138:1371–1381. doi: 10.1242/dev.058305. https://doi.org/10.1242/dev.058305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bacaj T, Tevlin M, Lu Y, Shaham S. Glia are essential for sensory organ function in C. elegans. Science. 2008;322:744–747. doi: 10.1126/science.1163074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Procko C, Lu Y, Shaham S. Sensory organ remodeling in Caenorhabditis elegans requires the zinc-finger protein ZTF-16. Genetics. 2012;190:1405–1415. doi: 10.1534/genetics.111.137786. https://doi.org/10.1534/genetics.111.137786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bargmann CI, Horvitz HR. Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science. 1991;251:1243–1246. doi: 10.1126/science.2006412. https://doi.org/10.1126/science.2006412. [DOI] [PubMed] [Google Scholar]
- 77.Peckol EL, Troemel ER, Bargmann CI. Sensory experience and sensory activity regulate chemosensory receptor gene expression in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2001;98:11032–11038. doi: 10.1073/pnas.191352498. https://doi.org/10.1073/pnas.191352498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Han Z, Boas S, Schroeder NE. Unexpected variation in neuroanatomy among diverse nematode species. Front Neuroanat. 2016;9:1–11. doi: 10.3389/fnana.2015.00162. https://doi.org/10.3389/fnana.2015.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schroeder NE, Androwski RJ, Rashid A, Lee H, Lee J, Barr MM. Dauer-specific dendrite arborization in C. elegans is regulated by KPC-1/Furin. Curr Biol. 2013;23:1527–1535. doi: 10.1016/j.cub.2013.06.058. https://doi.org/10.1016/j.cub.2013.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith CJ, Watson JD, Spencer WC, Brien TO, Cha B, Albeg A, Treinin M, Miller DM. Time-lapse imaging and cell-specific expression profiling reveal dynamic branching and molecular determinants of a multi-dendritic nociceptor in C. elegans. Dev Biol. 2010;345:18–33. doi: 10.1016/j.ydbio.2010.05.502. https://doi.org/10.1016/j.ydbio.2010.05.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. https://doi.org/10.1016/S0896-6273(00)81199-X. [DOI] [PubMed] [Google Scholar]
- 82.Li W, Kang L, Piggott BJ, Feng Z, Xu XZS. The neural circuits and sensory channels mediating harsh touch sensation in Caenorhabditis elegans. Nat Commun. 2011;2:315. doi: 10.1038/ncomms1308. https://doi.org/10.1038/ncomms1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cinar HN, Chisholm AD. Genetic analysis of the Caenorhabditis elegans pax-6 locus: roles of paired domain-containing and nonpaired domain-containing isoforms. Genetics. 2004;168:1307–1322. doi: 10.1534/genetics.104.031724. https://doi.org/10.1534/genetics.104.031724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keane J, Avery L. Mechanosensory inputs influence Caenorhabditis elegans pharyngeal activity via ivermectin sensitivity genes. Genetics. 2003;164:153–162. doi: 10.1093/genetics/164.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choe A, von Reuss SH, Kogan D, Gasser RB, Platzer EG, Schroeder FC, Sternberg PW. Ascaroside signaling is widely conserved among nematodes. Curr Biol. 2012;22:772–780. doi: 10.1016/j.cub.2012.03.024. https://doi.org/10.1016/j.cub.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gems D, McElwee JJ. Broad spectrum detoxification: the major longevity assurance process regulated by insulin/IGF-1 signaling? Mech Ageing Dev. 2005;126:381–387. doi: 10.1016/j.mad.2004.09.001. https://doi.org/10.1016/j.mad.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 87.Wolkow C, Hall DH. The Dauer Cuticle. Wormatlas. 2011 [Google Scholar]
- 88.Colella E, Li S, Roy R. Developmental and cell cycle quiescence is mediated by the nuclear hormone receptor co-regulator DIN-1S in the C. elegans dauer larva. Genetics. 2016;203:1763–1776. doi: 10.1534/genetics.116.191858. https://doi.org/10.1534/genetics.116.191858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Narbonne P, Roy R. Inhibition of germline proliferation during C. elegans dauer development requires PTEN, LKB1 and AMPK signalling. Development. 2006;133:611–619. doi: 10.1242/dev.02232. https://doi.org/10.1242/dev.02232. [DOI] [PubMed] [Google Scholar]
- 90.Nelson FK, Riddle DL. Functional study of the Caenorhabditis elegans secretory-excretory system using laser microsurgery. J Exp Zool. 1984;231:45–56. doi: 10.1002/jez.1402310107. https://doi.org/10.1002/jez.1402310107. [DOI] [PubMed] [Google Scholar]
- 91.Nelson FK, Albert PS, Riddle DL. Fine structure of the Caenorhabditis elegans secretory—excretory system. J Ultrastruct Res. 1983;82:156–171. doi: 10.1016/s0022-5320(83)90050-3. https://doi.org/10.1016/S0022-5320(83)90050-3. [DOI] [PubMed] [Google Scholar]
- 92.Schaedel ON, Gerisch B, Antebi A, Sternberg PW. Hormonal signal amplification mediates environmental conditions during development and controls an irreversible commitment to adulthood. PLoS Biol. 2012;10:e1001306. doi: 10.1371/journal.pbio.1001306. https://doi.org/10.1371/journal.pbio.1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Albert PS, Riddle DL. Mutants of Caenorhabditis elegans that form dauer-like larvae. Dev Biol. 1988;126:270–293. doi: 10.1016/0012-1606(88)90138-8. https://doi.org/10.1016/0012-1606(88)90138-8. [DOI] [PubMed] [Google Scholar]
- 94.Antebi A, Culotti JG, Hedgecock EM. daf-12 regulates developmental age and the dauer alternative in Caenorhabditis elegans. Development. 1998;125:1191–1205. doi: 10.1242/dev.125.7.1191. [DOI] [PubMed] [Google Scholar]
- 95.Ohkura K, Suzuki N, Ishihara T, Katsura I. SDF-9, a protein tyrosine phosphatase-like molecule, regulates the L3/dauer developmental decision through hormonal signaling in C. elegans. Development. 2003;130:3237–3248. doi: 10.1242/dev.00540. https://doi.org/10.1242/dev.00540. [DOI] [PubMed] [Google Scholar]
- 96.Li J, Brown G, Ailion M, Lee S, Thomas JH. NCR-1 and NCR-2, the C. elegans homologs of the human Niemann-Pick type C1 disease protein, function upstream of DAF-9 in the dauer formation pathways. Development. 2004;131:5741–5752. doi: 10.1242/dev.01408. https://doi.org/10.1242/dev.01408. [DOI] [PubMed] [Google Scholar]
- 97.Chitwood DJ, Lusby WR, Lozano R, Thompson MJ, Svoboda JA. Sterol metabolism in the nematode Caenorhabditis elegans. Lipids. 1984;19:500–506. doi: 10.1007/BF02534482. https://doi.org/10.1007/BF02534482. [DOI] [PubMed] [Google Scholar]
- 98.Matyash V, Entchev EV, Mende F, Wilsch-Bräuninger M, Thiele C, Schmidt AW, Knölker H-J, Ward S, Kurzchalia TV. Sterol-derived hormone(s) controls entry into diapause in Caenorhabditis elegans by consecutive activation of DAF-12 and DAF-16. PLoS Biol. 2004;2:e280. doi: 10.1371/journal.pbio.0020280. https://doi.org/10.1371/journal.pbio.0020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang RA. C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. https://doi.org/10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 100.Gottlieb S, Ruvkun G. daf-2, daf-16 and daf-23: genetically interacting genes controlling dauer formation in Caenorhabditis elegans. Genetics. 1994;137:107–120. doi: 10.1093/genetics/137.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vowels JJ, Thomas JH. Genetic analysis of chemosensory control of dauer formation in Caenorhabditis elegans. Genetics. 1992;130:105–123. doi: 10.1093/genetics/130.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. https://doi.org/10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- 103.Narbonne P, Roy R. Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature. 2009;457:210–214. doi: 10.1038/nature07536. https://doi.org/10.1038/nature07536. [DOI] [PubMed] [Google Scholar]
- 104.Cunningham KA, Bouagnon AD, Barros AG, Lin L, Malard L, Romano-Silva MA, Ashrafi K. Loss of a neural AMP-activated kinase mimics the effects of elevated serotonin on fat, movement, and hormonal secretions. PLoS Genet. 2014;10:17–20. doi: 10.1371/journal.pgen.1004394. https://doi.org/10.1371/journal.pgen.1004394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ailion M, Thomas JH. Isolation and characterization of high-temperature-induced dauer formation mutants in Caenorhabditis elegans. Genetics. 2003;165:127–144. doi: 10.1093/genetics/165.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.West-Eberhard MJ. Developmental plasticity and the origin of species differences. Proc Natl Acad Sci. 2005;102:6543–6549. doi: 10.1073/pnas.0501844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Viney ME, Gardner MP, Jackson JA. Variation in Caenorhabditis elegans dauer larva formation. Dev Growth Differ. 2003;45:389–396. doi: 10.1046/j.1440-169x.2003.00703.x. https://doi.org/10.1046/j.1440-169X.2003.00703.x. [DOI] [PubMed] [Google Scholar]
- 108.Hodgkin J, Doniach T. Natural variation and copulatory plug formation in Caenorhabditis elegans. Genetics. 1997;146:149–164. doi: 10.1093/genetics/146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nicholas WL, Dougherty EC, Hansen EL. Axenic cultivation of Caenorhabditis briggsae (Nematoda: Rhabditidae) with chemically undefined supplements; comparative studies with related nematodes. Ann N Y Acad Sci. 1959;77:218–236. https://doi.org/10.1111/j.1749-6632.1959.tb36902.x. [Google Scholar]
- 110.Staniland LN. Experiments on the control of chrysanthemum eelworm (Aphelenchoides ritzema-bosi, Schwartz) hot water treatment. Ann Appl Biol. 1950;37:11–18. https://doi.org/10.1111/j.1744-7348.1950.tb00946.x. [Google Scholar]
- 111.Staniland LN. Now we’ll try oils. Foley House; 1950. [Google Scholar]
- 112.Staniland LN. The principles of line illustration with emphasis on the requirements of biological and other scientific workers. Cambridge: Cambridge University Press; 1953. [Google Scholar]
- 113.Southey JF, Staniland LN. Observations and experiments on stem eelworm, Ditylenchus dipsaci (Kühn, 1857) Filipjev, 1936, with special reference to weed hosts. J Helminthol. 1950;24:145–154. https://doi.org/10.1017/S0022149X00019210. [Google Scholar]
- 114.Staniland L. The swarming of Rhabditid eelworms in mushroom houses. Plant Pathol. 1957;6:61–62. [Google Scholar]
- 115.Burr AH. The photomovement of Caenorhabditis elegans, a nematode which lacks ocelli. Proof that the response is to light not radiant heating. Photochem Photobiol. 1985;41:577–582. doi: 10.1111/j.1751-1097.1985.tb03529.x. https://doi.org/10.1111/j.1751-1097.1985.tb03529.x. [DOI] [PubMed] [Google Scholar]
- 116.Kiontke K, Sudhaus W. Ecology of Caenorhabditis species. In: Fitch DH, editor. Wormbook. The C. elegans Research Community; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Castelletto ML, Gang SS, Okubo RP, Tselikova AA, Nolan TJ, Platzer EG, Lok JB, Hallem EA. Diverse host-seeking behaviors of skin-penetrating nematodes. PLOS Pathog. 2014;10:e1004305. doi: 10.1371/journal.ppat.1004305. https://doi.org/10.1371/journal.ppat.1004305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ashton FT, Zhu X, Boston R, Lok JB, Schad GA. Strongyloides stercoralis: Amphidial neuron pair ASJ triggers significant resumption of development by infective larvae under host-mimicking in vitro conditions. Exp Parasitol. 2007;115:92–97. doi: 10.1016/j.exppara.2006.08.010. https://doi.org/10.1016/j.exppara.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hallem EA, Rengarajan M, Ciche TA, Sternberg PW. Nematodes, bacteria, and flies : A tripartite model for nematode parasitism. Curr Biol. 2007;17:898–904. doi: 10.1016/j.cub.2007.04.027. https://doi.org/10.1016/j.cub.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 120.Albarqi MMY, Stoltzfus JD, Pilgrim AA, Nolan TJ, Wang Z, Kliewer SA, Mangelsdorf DJ, Lok JB. Regulation of life cycle checkpoints and developmental activation of infective larvae in Strongyloides stercoralis by dafachronic acid. PLOS Pathog. 2016;12:e1005358. doi: 10.1371/journal.ppat.1005358. https://doi.org/10.1371/journal.ppat.1005358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ogawa A, Streit A, Antebi A, Sommer RJ. A conserved endocrine mechanism controls the formation of dauer and infective larvae in nematodes. Current Biology. 2009;19:67–71. doi: 10.1016/j.cub.2008.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]


