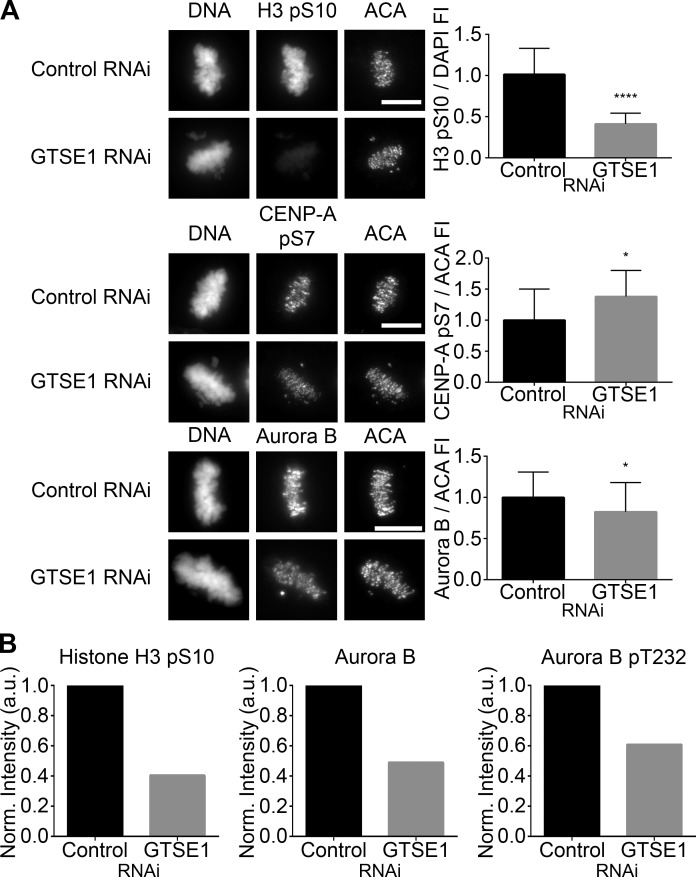
Figure 5.
GTSE1 modulates Aurora B kinase activity during mitosis. (A, left) Immunofluorescence of histone H3 pS10, CENP-A pS7, or Aurora B levels in metaphase HeLa cells transfected with either control or GTSE1 siRNA. Cells were coimmunostained for DNA and kinetochores. Images represent maximum-intensity projections. Histone H3 pS10, CENP-A pS7, and Aurora B levels are scaled equivalently in each panel. Bars, 10 µm. (Right) Fluorescence intensity quantification of chromosome-bound histone H3 pS10 and kinetochore-localized CENP-A pS7 and Aurora B immunofluorescence after control or GTSE1 siRNA transfection. Depletion of GTSE1 greatly reduced the levels of histone H3 pS10 on chromosome arms. In contrast, the level of pS7 on kinetochore-localized CENP-A was slightly increased, whereas the total amount of kinetochore-associated Aurora B was slightly decreased. Thus, GTSE1 depletion diminishes Aurora B activity on chromosome arms but increases Aurora B activity at kinetochores. SD is plotted for the mean from each condition. Histone H3 pS10 n = 20 cells, CENP-A pS7 n = 10 cells, and Aurora B n = 30 cells from two independent experiments for each condition. *, P < 0.05; ****, P < 0.0001. FI, fluorescence intensity. (B) Quantification of the chromosome-bound fractions from the immunoblot shown in Fig. S3 B. Histone H3 pS10, total Aurora B, and Aurora B pT232 levels were normalized to histone H3. Although the reduction in histone H3 pS10 levels by immunoblot were comparable to data obtained by immunofluorescence (A, top), in the absence of GTSE1, total chromosome-bound Aurora B levels were further reduced when compared with kinetochore-localized Aurora B (A, bottom), suggesting that in cells depleted of GTSE1, there is a greater loss of Aurora B from chromosome arms compared with Aurora B at kinetochores. Similarly, a fraction of Aurora B autophosphorylation activity was reduced in the absence of GTSE1.