Casanova and Winckler discuss Liu et al.’s recent finding that WDR91 coordinates Rab and phosphoinositide conversion during endosome maturation in neurons.
Abstract
Endosome maturation requires a coordinated change in the Rab GTPase and phosphoinositide composition of the endosomal membrane. In this issue, Liu et al. (2017. J. Cell Biol. https://doi.org/10.1083/jcb.201705151) identify WDR91 as a ubiquitous Rab7 effector that inhibits phosphatidylinositol 3-kinase activity on endosomes and is critical for endosome maturation, viability, and dendrite growth of neurons in vivo.
The endocytic pathway of eukaryotic cells consists of an ordered series of intracellular compartments (endosomes) with distinct biochemical compositions and biological functions. Although originally thought to be stable organelles, it is now well established that endosomes undergo a process of maturation, such that early endosomes mature to become late endosomes, which eventually either fuse with existing lysosomes or mature further to become lysosomes themselves (Scott et al., 2014). Endosome maturation is essential for the delivery of endocytosed cargos to lysosomes for degradation and for the termination of signaling by a wide variety of signaling receptors.
Rabs are small GTPases that control many aspects of membrane traffic, including the maturation of early endosomes to late endosomes (Wandinger-Ness and Zerial, 2014). Early endosomes are marked by Rab5, which regulates fusion of incoming vesicles with existing endosomes as well as between existing early endosomes. In contrast, late endosomes are marked by Rab7, which regulates their fusion with vesicles from the trans-Golgi network carrying lysosomal enzymes, attachment of late endosomes to microtubule motors that drive their migration toward the microtubule-organizing center, as well as their fusion with other late endosomes and with lysosomes. Endosome identity and function are also specified by the presence of distinct phosphoinositide species (Schink et al., 2016): early endosomes are rich in phosphatidylinositol 3-phosphate (PtdIns3P), whereas late endosomes contain phosphatidylinositol 3,5-bisphosphate (PtdIns(3,5)P2). During endosome maturation, Rab5 is replaced by Rab7 in a highly coordinated process referred to as Rab conversion. In this well-characterized process, Rab5 together with PtdIns3P recruits a complex containing the proteins Mon1/SAND1 and Ccz1/CCZ1 to early endosomes. This complex has two important functions. First, Mon1/SAND1 suppresses Rab5 activity by displacing its guanine nucleotide exchange factor (GEF), Rabex-5. Second, Ccz1/CCZ1 acts as a GEF for Rab7, leading to Rab7 activation. Rab7 then recruits TBC2, a Rab5-inactivating GTPase-activating protein that further reduces Rab5 activity, resulting in the dissociation of Rab5 effectors such as EEA1 and their replacement with Rab7 effectors that define late endosome function (Scott et al., 2014).
Although Rab conversion is relatively well understood, how phosphoinositide conversion occurs is still an open question. The enzymes generating and converting phosphoinositides are recruited to endosomes by activated Rab proteins and thereby create a lipid environment conducive to the recruitment of additional endosomal effectors. For instance, Rab5-GTP recruits the class III phosphatidylinositol 3-kinase (PI3K) complex (consisting of Beclin-1, Vps34, and Vps15) to early endosomes, resulting in high local levels of PtdIns3P. Interestingly, Rab7 also binds the Vps34 complex, yet late endosomes contain little PtdIns3P. To some extent, this can be explained by phosphorylation of PtdIns3P by the kinase PIKfyve, generating the PtdIns(3,5)P2 that characterizes late endosomes. PtdIns3P can also be dephosphorylated by endosomally localized myotubularin family (MTM) phosphatases to generate phosphatidylinositol (Schink et al., 2016). Pharmacological inhibition of PIKfyve or expression of catalytically inactive mutants causes dramatic swelling of endosomal compartments that appear to be late endosomes and lysosomes (Dove et al., 2009). Surprisingly, whereas knockdown of the myotubularins MTM1 and MTM2 leads to increased levels of PtdIns3P on early and late endosomes, respectively, it has not been reported to induce endosome swelling (Cao et al., 2008). Liu et al. (2016) recently showed that the suppressor of organelle fusion (SORF) proteins in Caenorhabditis elegans controls phosphoinositide conversion during endosome maturation. In this issue, Liu et al. now establish that WDR91, the mammalian homologue of SORF-1, is a Rab7 effector that controls phosphoinositide switching during endosome maturation and is essential to ensure the proper growth of dendrites in vivo and normal life span of the organism.
Interestingly, unlike PIKfyve and myotubularins, which function in PtdIns3P consumption, SORF/WDR proteins act to suppress PtdIns3P production during the maturation of early endosomes to late endosomes. Genetic loss of Vps18, a component of the HOPS tethering complex, results in dramatic defects in endosome/lysosome fusion in C. elegans. In a screen to identify suppressors of this phenotype, Liu et al. (2016) identified two genes, ZK563.5 and F52C9.1, which they called SORF-1 and -2. The products of these genes form a complex that is essential for phosphoinositide conversion in the transition from early to late endosomes. Deletion of either gene led to enlarged early endosomes in worm coelomocytes (analogous to macrophages) and impaired the transport of endocytosed cargo to lysosomes. The enlarged endosomes were found to contain elevated levels of PtdIns3P in their limiting membranes, suggesting that SORF-1 and SORF-2 somehow negatively regulate PtdIns3P levels on early endosomes (Liu et al., 2016). Consistent with this hypothesis, the authors found that SORF-1 and SORF-2 associated with the class III PI3K complex through an interaction with Beclin-1 and negatively regulated PI3K catalytic activity in vitro.
In C. elegans, the phosphoinositide conversion pathway appears to be uncoupled from Rab conversion, as PtdIns3P levels decrease normally in the absence of sand-1, rab-7, or tbc-2. Thus, despite the fact that the Mon1–Ccz1 pathway suppresses Rab5 activity and that Rab5 is essential for Vps34 activation on early endosomes, SORF proteins remain essential for timely PtdIns3P down-regulation during endosome maturation. Importantly, however, endosome maturation is not completely blocked in SORF mutants; rather, the rate of maturation is slowed by more than twofold. Presumably, this is because PIKfyve and myotubularins remain active in the absence of SORF proteins and can eventually reduce PtdIns3P levels to the point where endosome maturation can proceed.
Mammals express homologues of SORF-1 and SORF-2, called WDR91 and WDR81, respectively. As in nematodes, the two proteins were shown to form a complex that colocalized partially with both Rab5 and Rab7, consistent with a role in endosome maturation. Similarly, WDR91 and WDR81 coprecipitated with the Beclin-1–Vps15–Vps34 PI3K complex, and knockdown of either protein in HeLa cells resulted in increased PtdIns3P levels on enlarged early endosomes, accumulation of endocytosed cargos in early endosomes, and a significant delay in the delivery of cargos (EGFR and dextran) to lysosomes (Liu et al., 2016). In the current follow up paper, Liu et al. (2017) examine the role of mammalian WDR91/81 in more detail. They demonstrate that mammalian WDR91 interacts directly with Rab7-GTP and can therefore be considered a bona fide Rab7 effector. This is in contrast to the C. elegans proteins SORF-1 and SORF-2. The interaction with Rab7 is mediated by an extended C-terminal WD40 domain that is present in WDR91, but not in its C. elegans orthologue SORF-1. Accordingly, endogenous WDR91 and its partner WDR81 in mammalian cells associate with endosomes in a Rab7-dependent manner, and WDR91 mutants that cannot bind Rab7 remain diffusely cytosolic. Importantly, binding of Rab7 and Beclin-1 to WDR91 is not mutually exclusive, as Beclin-1 associates with an N-terminal coiled-coil domain (Fig. 1). Like its counterpart in C. elegans, WDR91 was shown to inhibit the PI3K activity of the Beclin-1–Vps15–Vps34 complex, although the mechanism of inhibition remains undefined. Given the critical role of Rab7 in endosome maturation, the discovery of a new Rab7 effector is a significant advance for the field.
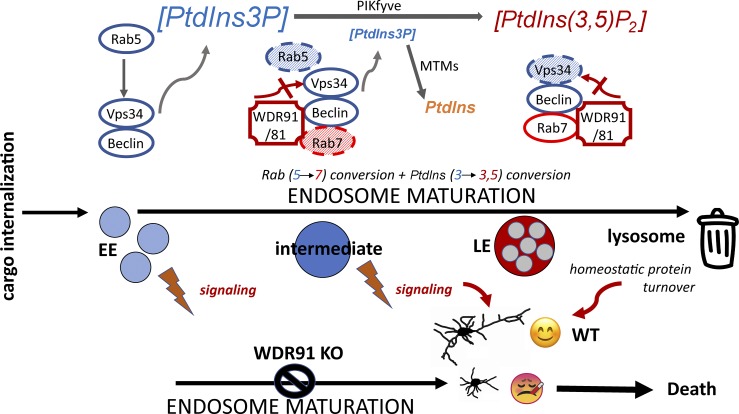
Figure 1.
The complexes that form during endosome maturation as Rabs and phosphoinositides are converted are shown. WDR91 binds both Rab7 and Beclin1 and can inhibit the activity of Vps34, thus reducing the levels of PtdIns3P on the endosomal membrane, which is necessary for endosome maturation.
What are the consequences of disrupting early endosome–late endosome maturation? As seen in the SORF and WDR mutants, endolysosomal compartments are profoundly disrupted, leading to enlarged endosomes that are no longer capable of transporting endocytosed cargo toward degradation. Failure of timely degradation is expected to lead to both detrimental changes in signaling cascades from activated receptors, as well as to the overall accumulation of undigested materials and disruption of protein homeostasis. Accumulated intermediates can become toxic to cells, and a large number of diseases are genetically linked to genes encoding components of the endosomal machinery responsible for regulating degradative flux (Wang et al., 2013). These genes include Rab7 itself, which has been linked to a neurodegenerative disorder called Charcot-Marie-Tooth disease (CMT subtype 2B). CMT is one of the most common inherited neurological disorders and results in neuropathy of peripheral motor and sensory axons. Mutations in PIKfyve have also been linked to forms of CMT, as well as to amyotrophic lateral sclerosis. Mutations in the myotubularins MTM1 and MTM2 are associated with CMT, as well as with X-linked myotubular myopathy. Interestingly, WDR81 has been linked genetically to a rare neurodegenerative disease in humans presenting with cerebellar hypoplasia and quadrupedal locomotion (Gulsuner et al., 2011). Endosome maturation is thus essential for long-term maintenance of long axon tracts in humans.
Given the neuropathological connection suggested by the WDR81 patients, this new paper from Liu et al. (2017) focused on the role of WDR91/81 in the brain. Global knockout of WDR91 was perinatally lethal; therefore, they generated a conditional WDR91 knockout mouse, driven by the promoter for the neural progenitor gene nestin (nestin-Cre). WDR91 is thus missing from the vast majority of central nervous system neurons early in development. So what are the phenotypes of the WDR91 knockout mouse? On a cellular level, cultured neurons derived from the WDR91 knockout brain show enlarged early endosomes similar to those found in C. elegans coelomocytes and in HeLa cells. These ubiquitously expressed proteins thus appear to play similar roles in all cell types investigated so far. The conditional knockout mouse has profound phenotypes at the organismal level, including smaller body size, greatly shortened life span, and a smaller brain. At the cellular level, dendrites are shorter, and there is increased apoptosis of neurons. Significantly, many of the described phenotypes do not manifest at birth and are not statistically significant at postnatal day 10 (P10) but only at P21. The brain thus appears to form mostly normally during development initially, and profound defects are only detected after the second week of life, a period where substantial growth of dendrites and synapse formation take place.
These new discoveries raise many intriguing questions. One of the remaining open questions is why dendrites initially grow normally and then fail to continue growing after P10 and why WDR91 knockout animals show such a profound shortening of life span. The slowing of overall degradative flux appears to cause sufficient proteostatic stress such that intrinsic apoptotic pathways are triggered, leading to cell death. In addition, it is possible that particular signaling cascades (such as brain-derived neurotrophic factor or other growth factors) exhibit changes in signal robustness or duration (Barford et al., 2017), such that continued dendrite growth is impaired. It will be interesting to investigate in the future how signaling by particular growth factors might be changed, how synapse function might be impaired, and whether recycling pathways or delivery of proteins to the cell surface via endosomes might be altered in the WDR91 knockout mouse. In addition, axon growth is also expected to be impaired, and it would be interesting to investigate the effects of loss of WDR91 on axons in the future. Endosomal cell biology is historically performed in cell culture models, but knockout mouse models are increasingly being generated. The generation of this mouse model is thus a welcome new tool for the field that will allow for a fuller exploration of the essential roles of endosome maturation during development and growth of the whole organism.
Acknowledgments
The authors declare no competing financial interests.
References
- Barford K., Deppmann C., and Winckler B.. 2017. The neurotrophin receptor signaling endosome: Where trafficking meets signaling. Dev. Neurobiol. 77:405–418. 10.1002/dneu.22427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C., Backer J.M., Laporte J., Bedrick E.J., and Wandinger-Ness A.. 2008. Sequential actions of myotubularin lipid phosphatases regulate endosomal PI(3)P and growth factor receptor trafficking. Mol. Biol. Cell. 19:3334–3346. 10.1091/mbc.E08-04-0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove S.K., Dong K., Kobayashi T., Williams F.K., and Michell R.H.. 2009. Phosphatidylinositol 3,5-bisphosphate and Fab1p/PIKfyve underPPIn endo-lysosome function. Biochem. J. 419:1–13. 10.1042/BJ20081950 [DOI] [PubMed] [Google Scholar]
- Gulsuner S., Tekinay A.B., Doerschner K., Boyaci H., Bilguvar K., Unal H., Ors A., Onat O.E., Atalar E., Basak A.N., et al. 2011. Homozygosity mapping and targeted genomic sequencing reveal the gene responsible for cerebellar hypoplasia and quadrupedal locomotion in a consanguineous kindred. Genome Res. 21:1995–2003. 10.1101/gr.126110.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Jian Y., Sun X., Yang C., Gao Z., Zhang Z., Liu X., Li Y., Xu J., Jing Y., et al. 2016. Negative regulation of phosphatidylinositol 3-phosphate levels in early-to-late endosome conversion. J. Cell Biol. 212:181–198. (published erratum appears in J. Cell. Biol. 2016. 212:739) 10.1083/jcb.201506081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Xing R., Jian Y., Gao Z., Ma X., Sun X., Li Y., Xu M., Wang X., Jing Y., et al. 2017. WDR91 is a Rab7 effector required for neuronal development. J. Cell Biol. 10.1083/jcb.201705151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schink K.O., Tan K.-W., and Stenmark H.. 2016. Phosphoinositides in Control of Membrane Dynamics. Annu. Rev. Cell Dev. Biol. 32:143–171. 10.1146/annurev-cellbio-111315-125349 [DOI] [PubMed] [Google Scholar]
- Scott C.C., Vacca F., and Gruenberg J.. 2014. Endosome maturation, transport and functions. Semin. Cell Dev. Biol. 31:2–10. 10.1016/j.semcdb.2014.03.034 [DOI] [PubMed] [Google Scholar]
- Wandinger-Ness A., and Zerial M.. 2014. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb. Perspect. Biol. 6:a022616 10.1101/cshperspect.a022616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Chan C.-C., Cherry S., and Hiesinger P.R.. 2013. Membrane trafficking in neuronal maintenance and degeneration. Cell. Mol. Life Sci. 70:2919–2934. 10.1007/s00018-012-1201-4 [DOI] [PMC free article] [PubMed] [Google Scholar]