Abstract
Background/Objectives
In animal models, a role in the regulation of energy expenditure (EE) has been ascribed to sphingolipids, active components of cell membranes participating in cellular signaling. In humans, it is unknown whether sphingolipids play a role in the modulation of EE and, consequently, influence weight gain. The present study investigated the putative association of EE and weight gain with sphingolipid levels in human skeletal muscle, a component of fat-free mass (the strongest determinant of EE), in adipose tissue, and in plasma.
Subjects/Methods
Twenty-four hour EE, sleeping metabolic rate (SMR), and resting metabolic rate (RMR) were assessed for 35 healthy Native Americans of Southwestern heritage (24 male; 30.2 ± 7.73 yr). Sphingolipid (ceramide, C; sphingomyelin, SM) concentrations were measured in skeletal muscle tissue, subcutaneous adipose tissue, and plasma samples. After 6.68 years (0.26 – 12.4 yr), follow-up weights were determined in 16 participants (4 females).
Results
Concentrations of C24:0, SM18:1/26:1, and SM18:0/24:1 in muscle were associated with 24h-EE (r = −.47, P = .01), SMR (r = −.59, P = .0008), and RMR (r = −.44, P = .01), respectively. Certain muscle sphingomyelins also predicted weight gain (e.g. SM18:1/23:1, r = .74, P = .004). For specific muscle sphingomyelins which correlated with weight gain and EE (SM18:1/23:0, SM18:1/23:1 and SMR, r = −.51, r = −.41, respectively, all P < 0.03; SM18:1/24:2 and RMR, r = −.36, P = 0.03), associations could be reproduced with SMR in adipose tissue (all r < −.46, all P < .04), though not in plasma.
Conclusions
This study provides preliminary, novel evidence that specific muscle and adipose tissue sphingolipid compounds are associated with EE and weight gain in Native Americans of Southwestern heritage. Further studies are warranted to investigate whether sphingolipids of different body compartments act in concert to modulate energy balance in humans.
Introduction
Energy expenditure (EE) is largely explained by its major determinants sex, age, fat mass (FM), fat-free mass (FFM), and ethnicity (1), with FFM accounting for the greatest part of its variance (2). Weight gain and its attendant increase in FFM and FM are associated with increased 24-hour EE (24h-EE) (3). Most importantly, relatively lower EE is a predictor of weight gain (4). In recent years, efforts to uncover additional influencing parameters of human EE have revealed a broad spectrum of factors, comprising inter-individual heterogeneity in response to diet (5), hormonal (6), and genetic determinants (7). With respect to the ongoing obesity epidemic (8), it is important to identify other regulatory players of EE as putative targets of therapeutic intervention in metabolic diseases.
As major constituents of lipid rafts involved in cellular stress response (9), sphingolipids are functionally active components of cell membranes, participating in a plethora of biological signaling pathways (10, 11). Ceramides and sphingomyelins are two central entities within this family of lipids and the hydrolysis of sphingomyelins for ceramide synthesis is thought to be an important reaction involved in cellular communication (12). Dysregulation of sphingolipid metabolism has repeatedly been associated with key aspects of the metabolic syndrome, such as β-cell dysfunction (13), insulin resistance (14), and cardiovascular disease (15). Additionally, there is growing evidence that EE could be modulated by sphingolipid messaging. In mouse models, the reduction of plasma and adipose tissue ceramide levels lead to elevated EE (16, 17). Suggestive of an underlying mechanism of EE regulation, inhibition of de novo ceramide synthesis resulted in induction of mitochondrial uncoupling protein 3 (UCP3) in white adipose tissue (WAT) (16). Interestingly, in humans, almost no UCP3 is expressed in WAT (18). In line with a possible effect of sphingolipids on energy balance regulation, it has previously been described that, in a murine model, skeletal muscle sphingolipids may alter mitochondrial respiration, therefore influencing energy balance (19).
It is possible that sphingolipids in WAT affect other tissues more directly involved in energy homeostasis. In rodents, the central administration of ceramides mediated stress to the hypothalamic endoplasmic reticulum, consequently depressing the expression of UCPs in brown adipose tissue (BAT), thus reducing EE and leading to weight gain (20).
Such studies examining the metabolic relevance of sphingolipids in body compartments contributing considerably to rodent metabolism and lipid messaging, here led us to investigate the major determinant of EE in humans, FFM (2), in comparison to FM and plasma. Therefore, we hypothesized that a summary measure of skeletal muscle sphingolipids (as identified by principal components analyses) would be associated with EE in humans, and allow us to further identify specific sphingolipids of interest. Given such an association, we further explored the possible correlation of sphingolipids with weight gain in a smaller study cohort with longitudinal weight data. Since animal data point to a similar role of sphingolipids in different body compartments, we also investigated whether, compared to muscle, these lipids are unidirectionally associated with EE measures in adipose tissue and plasma.
Subjects and Methods
Subjects and Clinical Assessment
Data from 35 Native American individuals of ≥ half Southwestern heritage who between March, 1995 and May, 2008 participated in an ongoing study to identify risk factors for diabetes and weight gain was included in the present study (table 1) (4). Consented, healthy volunteers were admitted to the clinical research unit. For information on basic baseline metabolic assessment and skeletal muscle and adipose tissue biopsies, please see Supplementary Information. Only non-diabetic individuals (21) were included in this analysis. Measurements of sphingolipid content in skeletal muscle, adipose tissue, and fasting plasma (collected during an oral glucose tolerance test) were carried out in a single set of analyses at the Department of Pharmacology, University of California Irvine, Irvine, CA, USA (Dr. D. Piomelli) using liquid chromatography and mass spectrometry (accuracy: coefficient of variation, CV, 0.16 – 4.82%) (22, 23). Measurements of EE were taken during the same stay during which biopsies, anthropometry, and assessment of glucose tolerance were performed (see Metabolic Assessment).
Table 1.
Study population: demographic characteristics
| Study participants |
Subjects with follow-up data
for body weight |
|||||
|---|---|---|---|---|---|---|
| All subjects1, 2 (n = 35) |
Male (n = 24) |
Female (n = 11) |
All subjects2, 3 (n = 16) |
Male (n = 12) |
Female (n = 4) |
|
|
|
|
|||||
| Age, yr | 30.2 ± 7.73 | 30.4 ± 8.34 | 29.6 ± 6.54 | 27.9 ± 7.59 | 27.8 ± 8.48 | 28.4 ± 4.89 |
| Body weight, kg | 92.4 ± 17.2 | 92.7 ±17.6 | 91.7 ± 17.0 | 95.7 ± 17.6 | 91.4 ± 16.2 | 108.6 ± 17.0 |
| Body mass index, kg/m2 | 32.2 ± 5.22 | 30.7 ± 4.45 | 35.4 ± 5.52 | 33.2 ± 6.51 | 30.6 ± 4.33 | 41.2 ± 5.62 |
| Body fat, % | 32.3 ± 7.70 | 28.1 ± 4.79 | 41.4 ± 3.85 | 32.8 ± 8.29 | 29.3 ± 5.95 | 43.3 ± 3.99 |
| Fasting plasma glucose, mg/dl | 86.7 ± 10.4 | 85.3 ± 11.7 | 89.7 ± 6.1 | 83.9 ± 10.4 | 81.6 ± 10.4 | 90.8 ± 7.97 |
| 2h-plasma glucose, mg/dl | 128.0 ± 29.2 | 120.9 ± 27.7 | 143.5 ± 27.5 | 121.8 ± 31.9 | 119.8 ± 33.5 | 127.8 ± 30.1 |
| Fat mass, kg | 30.2 ± 9.89 | 26.6 ± 8.35 | 38.1 ± 8.54 | 32.2 ± 12.3 | 27.3 ± 8.91 | 47.0 ± 8.49 |
| Fat-free mass, kg | 62.2 ± 11.6 | 66.1 ± 10.2 | 53.6 ± 9.73 | 63.4 ± 9.27 | 64.1 ± 9.30 | 61.5 ± 10.3 |
| Glucose tolerance status4 | ||||||
| Normal glucose regulation | 23 (65.7%) | 20 (83.3%) | 3 (27.3%) | 12 (75%) | 10 (83.3%) | 2 (50%) |
| Impaired glucose regulation | 12 (34.3%) | 4 (16.7%) | 8 (72.3%) | 4 (25%) | 2 (16.7%) | 2 (50%) |
| Energy intake, kj/d5 (kcal/d) |
9402 ± 997 (2246 ± 238) |
9603 ± 949 (2294 ± 227) |
8970 ± 1029 (2143 ± 246) |
9536 ± 976 (2278 ± 233) |
9527 ± 1110 (2276 ± 265) |
9567 ± 470 (2286 ± 112) |
| 24h-respiratory quotient6 | 0.86 ± 0.04 | 0.86 ± 0.03 | 0.85 ± 0.06 | 0.86 ± 0.03 | 0.87 ± 0.03 | 0.85 ± 0.03 |
| 24h-energy expenditure,
kJ/d (kcal/d) |
9642 ± 1007 (2304 ± 241) |
9946 ± 682 (2376 ± 163) |
8992 ± 1319 (2148 ± 315) |
9847 ± 796 (2352 ± 190) |
9788 ± 728 (2338 ± 174) |
10052 ± 1335 (2401 ± 319) |
| Sleeping metabolic rate,
kJ/d (kcal/d) |
7036 ± 849 (1682 ± 203) |
7203 ± 846 (1721 ± 202) |
6601 ± 768 (1577 ± 184) |
6992 ± 612 (1671 ± 146) |
6880 ± 638 (1644 ± 153) |
7348 ± 402 (1764 ± 96) |
| Awake-and-fed thermogenesis,
kJ/d (kcal/d)7 |
2075 ± 689 (496 ± 165) |
2155 ± 766 (515 ± 183) |
1867 ± 432 (446 ± 103) |
2073 ± 452 (495 ± 108) |
2007 ± 490 (480 ± 117) |
2307 ± 230 (551 ± 55) |
| Resting metabolic rate,
kJ/d (kcal/d) |
7823 ± 1091 (1877 ± 263) |
8182 ± 1041 (1963 ± 249) |
7040 ± 756 (1690 ± 183) |
7853 ± 1039 (1884 ± 249) |
8073 ± 1061 (1939 ± 256) |
7192 ± 749 (1725 ± 180) |
| Carbohydrate oxidation,
kJ/d (kcal/d)8 |
4621 ± 1456 (1104 ± 348) |
5117 ± 889 (1222 ± 213) |
3701 ± 1901 (884 ± 454) |
5087 ± 777 (1215 ± 186) |
5307 ± 834 (1268 ± 199) |
4536 ± 28.6 (1084 ± 6.00) |
| Fat oxidation, kJ/d8 (kcal/d) |
3478 ± 1593 (831 ± 381) |
3326 ± 993 (795 ± 237) |
3760 ± 2433 (898 ± 581) |
3502 ± 1290 (839 ± 308) |
3229 ± 1152 (772 ± 275) |
4536 ± 1837 (1000 ± 439) |
All data reported as frequency (percentage) or mean ± SD.
Only healthy, non-diabetic (2h-plasma glucose < 200 mg/dl) individuals between 18 – 55 years old were included. Student’s t-test assured no difference in sphingolipid concentrations between Native Americans of full versus half Southwestern heritage, thus data were analyzed in the whole group of Native Americans.
Maximum sample size reported. May differ by phenotyping due to data availability (see Subjects and Clinical Assessment).
Median follow-up time: 6.68 years (0.26 – 12.4 years) with a body weight gain of 4.77 ± 13.2 kg (P < 0.05, equivalent to + 7.05 ± 19.1% compared to initial body weight) and an annual weight gain of 0.92 ± 2.84%.
For normal glucose regulation, fasting plasma glucose < 100 mg/dl and 2h-plasma glucose < 140 mg/dl, impaired glucose regulation at fasting plasma glucose 100 – 126 mg/dl and/or 2h-plasma glucose 140 – 199 mg/dl, in accordance with the American Diabetes Association guidelines (1).
Meals served at 8 AM, 11 AM, 4 PM, and 7 PM. Total energy intake during a 24-hour stay in a respiratory chamber. The intercept of the regression line between EE and SPA values measured from 11 AM till 1 AM provided the EE in the inactive state.
Awake-and-fed thermogenesis (AFT) (2), reflective of an individual’s thermogenesis from the sleeping state to the non-active, awake and fed state, and derived from the difference between EE in the inactive state and SMR.
Twenty-four hour respiratory quotient (24h-RQ), derived from the ratio of 24-hour carbon dioxide production and oxygen consumption (l), was extrapolated from each 15-minute time period to 24-hours.
After accounting for all-day urinary nitrogen excretion as a measure of protein oxidation, 24-hour fat and carbohydrate oxidation rates were calculated from the 24h-RQ. A maximum of 35 subjects were analyzed for possible associations with RMR, and 34, 30, and 27 volunteers for associations with 24h-EE and 24h-RQ, SMR, and AFT, respectively. Population characteristics are reported as mean ± SD.
1. Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Journal of diabetes investigation. 2010;1(5):212–28.
2. Piaggi P, Krakoff J, Bogardus C, Thearle MS. Lower “awake and fed thermogenesis” predicts future weight gain in subjects with abdominal adiposity. Diabetes. 2013;62(12):4043–51.
Subjects also participated in a longitudinal study of health during which they were invited for examinations approximately every two years at which basic anthropometrics and an OGTT were performed (24). Individuals who had developed diabetes (n = 6) were excluded from follow-up, which took place from January, 2003 until December, 2007. For 16 individuals, follow-up weight at the subjects’ last exam was used for calculation of weight change (see Statistical Analyses).
Pregnancy and/or lactation at time of first and follow-up visit were excluded and no information about pregnancy and lactation between the visits was available.
Selection criteria for studied subject groups along with sensitivity analyses are presented in Supplementary Information.
Both studies (clinicaltrials.gov, NCT00340132, NCT00339482) were approved by the Institutional Review Board of the National Institute of Diabetes and Digestive Kidney Diseases.
Metabolic Assessment
Measurements of 24h-EE were performed by whole-room indirect calorimetry (2). Briefly, the mean EE of all 15-minute sampling intervals between 8 AM and 7:45 AM of the following day were extrapolated to calculate 24h-EE. Sleeping metabolic rate (SMR) was calculated as the mean of all 15-minute periods for which spontaneous physical activity (SPA), determined using radar (2), had been less than 1.5% in the time period between 11:30 PM and 5 AM of the following day (25). AFT, 24-hour respiratory quotient (24h-RQ), 24h-lipid and carbohydrate oxidation were calculated (see legend of table 1). Resting metabolic rate (RMR) was measured by a ventilated hood system in the morning after an overnight fast, and was calculated as the mean EE during a 40-minute period in an awake, motionless state, and extrapolated to 24-hours. CVs for the repeated assessment of 24h-EE, SMR, and RMR were previously reported to be 2.4%, 3.7%, and 4.7%, respectively (2).
Statistical Analyses
Statistical analyses were performed with SAS Enterprise Guide 7.1 (SAS Institute, Inc., Cary, NC, USA) and normal distribution of data was assured. General linear models were used to calculate residuals of metabolic measurements after adjustment for their major known determinants (4). Pearson’s correlation was reported. Alpha-level was set to 0.05. To assess associations with weight change, the relative amount of annual weight change ([weight at follow up visit – weight at first visit]/weight at first visit * 100/years of follow-up; %Δwt/yr) was calculated.
As we expected a large number of identified sphingolipid moieties, principal component analysis (PCA) was performed to reduce dimensionality of data by summarizing the global variance of all skeletal muscle sphingolipid measurements and to identify factors of correlated, clustered variables. Specifically, the principal components (PCs, or factors) were calculated to yield the best linear combinations of observations (supplemental figures 1 and 2). The PCs with the highest variance as determined by the eigenvalues > 1.0 (supplemental figure 2) were used in subsequent analyses.
Significant skeletal muscle PCs were correlated with measurements of EE and weight change. The metabolic contribution of individual sphingolipids was investigated only for those lipids which strongly correlated (r > 0.6) with a PC which had demonstrated an association with that variable.
Results
Subjects
Following biopsy, weights were available for 16 subjects after a mean follow-up time of 6.7 years (0.26 – 12.4 years). On average, body weight had increased by 4.8 kg ± 13.2 kg with an annual change of 0.9% ± 2.8% and no difference comparing sexes (P = 0.15).
Sphingolipid content of biological samples and correlations of sphingolipids with anthropometric measures are reported (supplemental tables 6–8, supplemental figures 3–5).
Skeletal Muscle Tissue Sphingolipid Concentrations
PCA of skeletal muscle sphingomyelin concentrations identified two independent factors (supplemental table 1, supplemental figure 1), accounting for 46% and 22% of the total variance of all sphingomyelins. Both factors were introduced into linear models for correlation analyses with EE measurements and %Δwt/yr (table 2). SM-factor 1 was positively associated with %Δwt/yr (figure 1B) but not with residual 24h-EE, SMR (figure 1A), or RMR (all P > 0.05). The results were similar with removal of the outlier in the weight change analysis. SM-factor 2 was negatively associated with residual SMR (figure 1C; β = −60.4 kcal/d per SD change in factor 2, SE 29.7, P = 0.05), accounting for an additional 7% of its variance. For SM-factor 2, no association was seen with %Δwt/yr (figure 1D), residual 24h-EE, RMR, and AFT (all P > 0.05). Twenty-four hour-RQ and oxidation rates did not correlate with any factor (all P > 0.05). PCA of skeletal muscle ceramide concentrations identified one factor, accounting for 41% of the total variance of all ceramides. All ceramide concentrations clustered to a high degree with the computed factor (supplemental table 2). It in turn exhibited a negative correlation with 24h-EE (figure 1E), accounting for 5% of its variance (β = −69.9 kcal/d, CI −102.92 to −36.9 kcal/d), but no association with weight change (figure 1F), residual SMR, RMR, AFT, 24h-RQ, and oxidation rates.
Table 2.
Results of correlation analyses of SM-factors 1 and 2 and C-factor generated from PCA with EE and the relative amount of annual weight change.
| 24h-EE (kcal/d) | SMR (kcal/d) | RMR (kcal/d) | AFT (kcal/d) | %Δwt/yr | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Factors | n | r | P | n | r | P | n | r | P | n | r | P | n | r | P |
| SM-factor 1 | 34 | −0.16 | 0.37 | 30 | −0.32 | 0.09 | 35 | −0.26 | 0.14 | 26 | 0.04 | −0.31 | 15 | 0.65 | 0.02 |
| SM-factor 2 | 34 | 0.01 | 0.93 | 30 | −0.36 | 0.05 | 35 | −0.15 | 0.40 | 26 | 0.84 | 0.12 | 15 | −0.20 | 0.51 |
| C-factor | 31 | −0.37 | 0.04 | 29 | −0.23 | 0.23 | 35 | −0.13 | 0.46 | 27 | −0.23 | 0.25 | 16 | 0.50 | 0.08 |
Significant results are highlighted in bold.
Abbreviations: 24h-EE, 24-hour energy expenditure; SMR, sleeping metabolic rate; RMR, resting metabolic rate; AFT, awake-and-fed thermogenesis; %Δwt/yr, relative amount of annual weight change; C, ceramide; SM, sphingomyelin.
Figure 1.
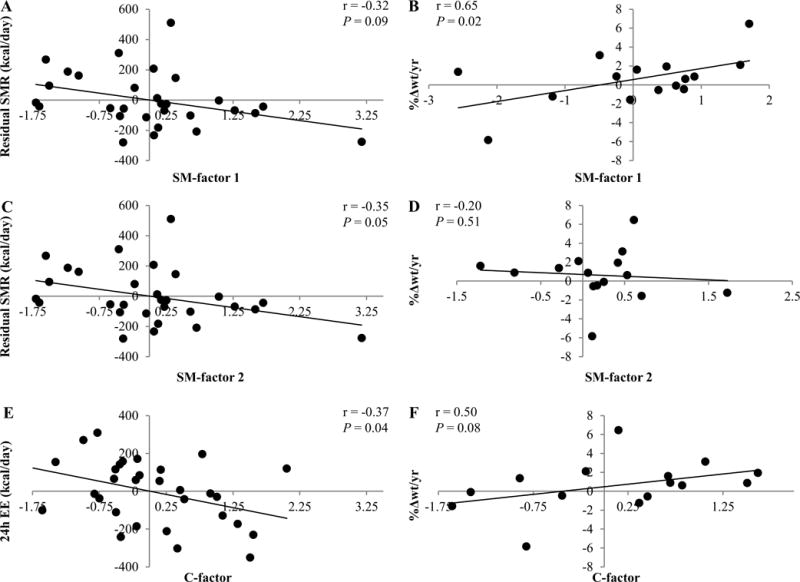
Correlation of principal component analysis (PCA) factors with the residual amount of energy expenditure (EE) measurements and the relative amount of annual weight change (%Δwt/yr). Twenty-four hour EE was adjusted for age, sex, fat mass (FM), fat-free mass (FFM), and physical activity. Sleeping metabolic rate (SMR) adjustments included age, sex, FM, and FFM. Sex and age were used as covariates for %Δwt/yr. Correlations of sphingomyelin factor 1 (SM-factor 1) with the residual amount of SMR and %Δwt/yr are shown in A and B, respectively. Correlations of sphingomyelin factor 2 (SM-factor 2) with the residual amount of SMR and %Δwt/yr are shown in C and D, respectively. Correlations of ceramide factor (C-factor) with the residual amount of 24h-EE and %Δwt/yr are shown in E and F, respectively. Pearson’s correlation and P-value are reported. Sensitivity analysis excluded the study volunteer who lost approximately 6% body weight per year: This nominally affected the P-value for the association of SM-factor 1 with %Δwt/yr (r = 0.53, P = 0.08). Given the lack of association between weight change per year and initial weight (r = −0.34, P = 0.20), we performed the analysis using weight change per year (kg/yr) only, yielding similar results for principal components.
With respect to a cutoff of r > 0.60 for correlation with sphingolipid PCs, sphingomyelins and ceramides were introduced into correlation analyses with EE measurements and %Δwt/yr (figure 2, supplemental table 3). Strong correlations were found for SM18:1/23:0, SM18:1/23:1, and SM18:1/26:1 levels with residual SMR (−0.59 ≤ r ≤ −0.41; 0.001 ≤ P ≤ 0.03) and %Δwt/yr (0.55 ≤ r ≤ 0.74; 0.004 ≤ P ≤ 0.05; figure 3A, C, E). SM18:1/23:1 accounted for 8% (β = −335.3 kcal/d per tenfold change, SE 145.1, P = 0.03), SM18:1/23:0 explained 12% (β = −38.6 kcal/d per tenfold change, SE 12.7, P = 0.01), and SM18:1/26:1 explained 16% (β = −434.6 kcal/d per tenfold change, SE 111.9, P = 0.001) of the additional variance of SMR (figure 4A, B, C). In line with the negative association of these sphingomyelins with residual SMR, these lipids associated positively with %Δwt/yr (figure 3B, D, F). Additionally, SM18:1/20:0 and SM18:0/18:0 showed negative associations with residual SMR (r = −0.5 and −0.45; P = 0.01 and 0.02, respectively). SM18:1/24:2 and SM18:0/24:1 (β = −0.02 kcal/d per tenfold change, SE 0.01, P = 0.01) were negatively correlated with residual RMR (r =−0.36 and −0.44; P = 0.03 and 0.01, respectively), with SM18:0/24:1 explaining 5% of the measure’s remaining variance. Consistent with its negative associations with residual RMR, skeletal muscle tissue concentrations of SM18:1/24:2 were positively associated with %Δwt/yr (r = 0.55; P = 0.05).
Figure 2.
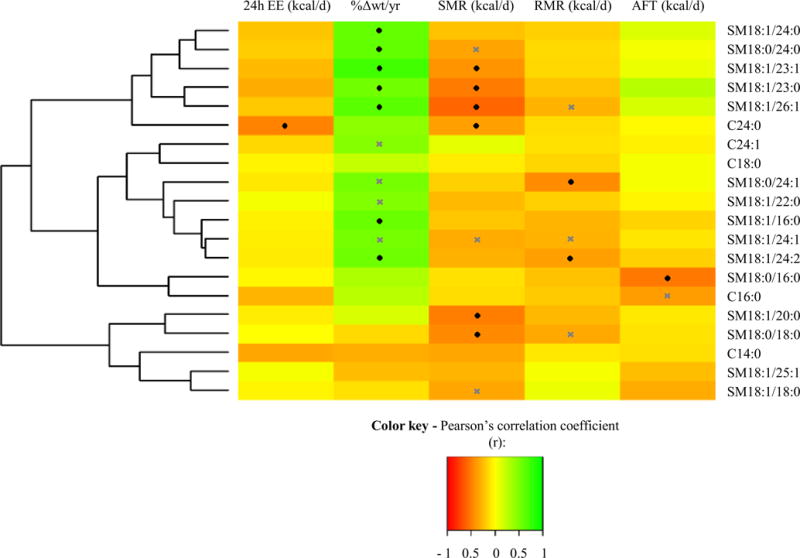
Heatmap for correlation analyses of sphingolipid concentrations in skeletal muscle tissue with residuals of EE measurements and the relative amount of annual weight change (%Δwt/yr). Twenty-four hour EE (24h-EE) was adjusted for age, sex, fat mass (FM), fat-free mass (FFM), and physical activity. Sleeping metabolic rate (SMR) and resting metabolic rate (RMR) were adjusted for sex, age, FM, and FFM. Awake-and-fed thermogenesis (AFT) was adjusted for age, sex, percentage of body fat, and fasting glucose concentrations (obtained from OGTT). Sex and age were used as covariates for %Δwt/yr. Only those sphingolipids which showed strong correlations (r > 0.6) with previously calculated sphingolipid factors were introduced into analyses. Pearson’s correlation (values reported in supplemental table 3) is visualized as indicated by the color key, red being correlation of −1.0 and green being correlation of 1.0. Significant correlations are highlighted by a black diamond, borderline significant correlations (0.10 ≥ P > 0.05) by a grey cross. Two major groups and three major subdivisions are visualized by the dendrogram. The first subdivision is driven by sphingomyelins which largely associate with %Δwt/yr and SMR, whereas the second subdivision includes sphingolipids which barely correlate with %Δwt/yr. The last major subdivision includes sphingolipids which do not associate with %Δwt/yr at all. Abbreviations: C, ceramide; SM, sphingomyelin.
Figure 3.
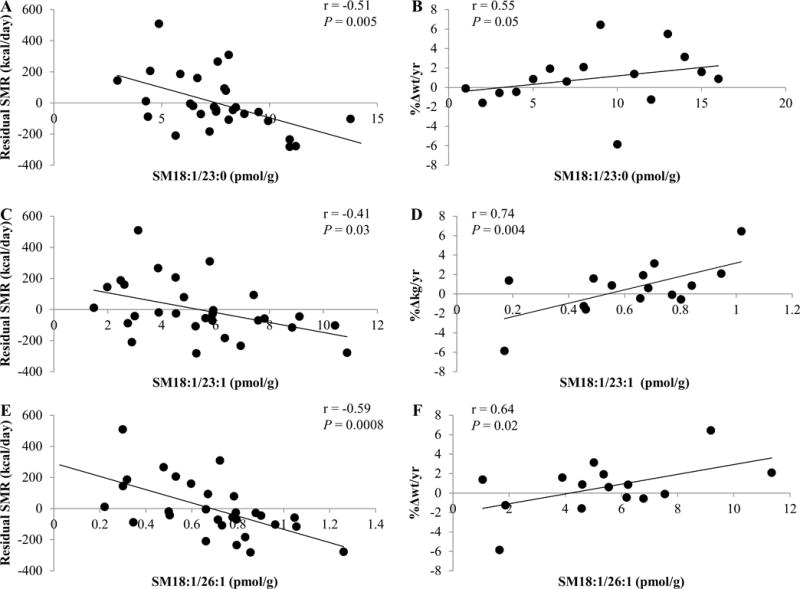
Correlation of SM18:1/23:0 (A, B), SM18:1/23:1 (B, C), and SM18:1/26:1 with the residual amount of sleeping metabolic rate (SMR) after adjustment for age, sex, fat mass (FM), fat-free mass (FFM), and weight change (%Δwt/yr). Sex and age were used as covariates for %Δwt/yr. Even after excluding the study volunteer who lost approximately 6% body weight per year, SM18:1/23:0 and SM18:1/26:1 tended to correlate with the relative amount of annual weight change (%Δwt/yr, r = 0.53, P = 0.08; r = 0.53, P = 0.08, respectively). SM18:1/23:1 correlated with %Δwt/yr (r = 0.64, P = 0.02). Of note, %Δwt/yr for correlation analyses with sphingolipids was used under the assumption that a linear relationship of initial weight (kg) and weight change per year (kg/yr) exists. Exploration of this did not support this assumption in the present study cohort (r = −0.34, P = 0.20). Thus, weight change per year (kg/yr) was correlated with sphingolipid moieties. Except for SM18:1/23:0 (r = 0.37, P = 0.21), all previous associations remained significant: SM18:1/23:1, and SM18:1/26:1 correlated with kg weight change per year (r = 0.56, P = 0.05; r = 0.66, P = 0.01, respectively). Therefore, results for associations with %Δwt/yr are reported. Pearson’s correlation and P-value are reported. Abbreviation: SM, sphingomyelin.
Figure 4.
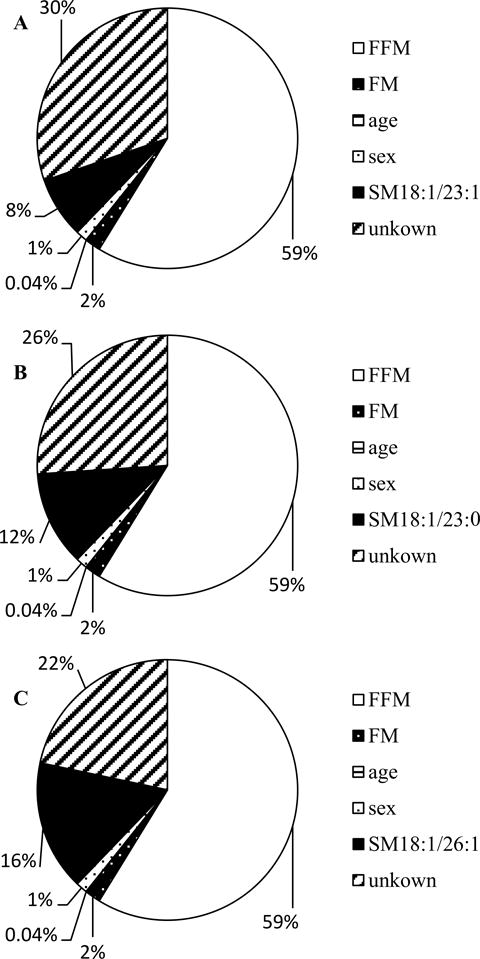
Variance of sleeping metabolic rate (SMR) explained by its major known determinants (fat mass, FM; fat-free mass, FFM; age, and sex), SM18:1/23:1 (A) and SM18:1/23:0 (B), and SM18:1/26:1. Abbreviation: SM, sphingomyelin.
Interestingly, SM18:1/16:0, SM18:1/24:0, and SM18:0/24:0, which did not correlate with EE measurements (all P > 0.05), were also associated with %Δwt/yr (figure 2, supplemental table 3; 0.58 ≤ r ≤ 0.61; 0.03 ≤ P ≤ 0.04).
Notably, only sphingomyelins for which an association was found with EE and %Δwt/yr are components of SM-factor 1 (supplemental table 1, figure 2).
Skeletal muscle tissue concentration of C24:0 associated negatively with residual 24h-EE (β = −663.9 kcal/d per tenfold change, SE 228.6, P = 0.007) and residual SMR (β = −519.7 kcal/d per tenfold change, SE 255.3, P = 0.05). C24:0 alone explained 6% and 5% of the additional variance of 24h-EE and SMR, respectively. Muscular ceramides did not correlate with residual RMR or %Δwt/yr (all P > 0.05).
In 34 individuals, SM18:1/23:0 correlated negatively with residual 24h-RQ (r = −0.39; P = 0.02; β = −0.003 per tenfold change, SE 0.002, P = 0.02), explaining an additional 8% of this measure’s variance. SM18:1/23:0 associated negatively with carbohydrate oxidation (r = −0.42; P = 0.02; β = −45.0 kcal/d per tenfold change, CI −83.0 to −7.01 kcal/d) and accounted for 10% of its additional variance. No correlations were found for sphingolipids and fat oxidation (all P > 0.05).
SM18:0/16:0 associated negatively with AFT (figure 2; r = −0.52; P = 0.01; β = −354.6 kcal/d per tenfold change, SE 117.7, P = 0.01), accounting for 19% of its additional variance.
Non-Skeletal Muscle Sphingolipid Concentrations
For subcutaneous adipose tissue and plasma, those sphingolipid moieties which showed a significant association with EE measurements or %Δwt/yr in skeletal muscle tissue were introduced into correlation analysis (supplemental table 4). Largely consistent with results in skeletal muscle, adipose SM18:1/23:0 (β = −333.7 kcal/d per tenfold change, SE 124.7, P = 0.01), SM18:1/23:1, SM18:1/24:2, and SM18:0/24:1 were strongly, negatively associated with residual SMR (0.39 ≤ r ≤ 0.46; 0.01 ≤ P ≤ 0.04). An additional 7% of the variance of SMR was explained by SM18:1/23:0 alone. Correlation analyses of sphingolipids with %Δwt/yr, 24h-RQ, and oxidation rates did not yield significant results (all P > 0.05).
For those sphingomyelin moieties which showed an association with residual SMR in skeletal muscle and subcutaneous adipose tissue (supplemental table 3 and 4), z-scores were calculated, and a summary score across these tissues was correlated with residual SMR. Summary scores associated with the residual amount of SMR after adjusting for age, sex, FM, and FFM (−0.66 ≤ r ≤ −0.54; 0.01 ≤ P ≤ 0.03). Of note, pooled sphingomyelin concentrations expressed stronger associations with residual SMR than adipose or skeletal muscle concentrations alone.
In plasma, no specific confirmatory associations were found.
Comparing sphingolipid concentrations in different tissues and plasma, associations were found (supplemental table 5). Notably, SM18:1/23:0 concentrations, which correlated positively in adipose and skeletal muscle tissue, associated negatively with residual SMR in both tissues and were linked to %Δwt/yr (figure 3, supplemental tables 3–5).
Discussion
In a cohort of Native Americans of Southwestern heritage, we found that specific sphingolipid compounds measured in skeletal muscle were determinants of EE, accounting for a considerable amount of its unexplained residual variance. For instance, SM18:1/26:1 accounted for nearly 16% and one of the PCA generated factors accounted for 7% of the remaining variance in SMR. In comparison, FFM, the strongest determinant of EE (1), alone explains 59% of the variance for the same measurement. Moreover, some of the here presented sphingomyelins were associated with weight gain. For those sphingomyelin moieties that correlated with EE and weight gain in muscle, we were able to largely confirm the associations with EE in adipose tissue. Pooled sphingomyelin concentrations for skeletal muscle and adipose tissue expressed strong correlations with EE. Furthermore, sphingomyelin and ceramide concentrations in plasma, muscle, and adipose tended to correlate. The strength of associations, magnitude of explained variance for EE measurements, and presented commonalities across body compartments observed in these explorative analyses suggest that candidate sphingomyelins might be involved in the regulation of EE in Native Americans of Southwestern heritage.
Recent studies have stated that sphingolipids participate in the modulation of EE as effectors of thermogenic mechanisms in mice (16, 17, 20). Driven by the influence of sphingolipids on mitochondrial heat production, current knowledge of possible underlying mechanisms focuses on adipocytes as the targeted cell type by those lipid messengers (16, 20). However, whether sphingolipids have similar roles in tissues that are stronger determinants of EE, such as FFM, is not clear. Therefore, the investigation of the skeletal muscle sphingolipid content with respect to EE offers a new approach to the topic, as does the inclusion of human samples and human EE measurements. In our study, the identification of several skeletal muscle sphingolipids as strong determinants of human EE, and the comparison of their effects with those in adipose tissue and plasma provide novel insight into the physiology of lipid-mediated EE regulation. Candidate muscle sphingolipids in humans not only account for a big portion of the yet unexplained variation in EE, but may be involved in the regulation of body weight change over time.
Studies on the physiology of sphingolipids have shown that, in rodents, the knockout of ceramide synthesizing enzyme CerS6 (CerS6Δ/Δ) leads to a reduction of C16:0 in WAT, BAT, and hepatocytes, although not changing concentrations in skeletal muscle (17). However, CerS6Δ/Δ mice had higher EE rates consistent with our findings of a negative association between specific ceramides and EE (17), and, previously, inhibition of de novo sphingolipid synthesis increased expression of UCP3 in WAT possibly affecting thermogenesis (16). Ceramides are a building block for sphingomyelin synthesis, and the latter can be hydrolyzed yielding ceramides. Given this interchangeability, cellular functions of ceramides – possibly including their influence on whole-body EE – may partially be ascribed to sphingomyelins (10, 11).
Whether changes in sphingolipid concentrations and associated alterations in UCP expression described in WAT are also occurring in muscle and thereby affecting EE is not clear. However, in humans, UCP3 is expressed in skeletal muscle tissue and, like UCP1, can also be found in BAT (18). Skeletal muscle is part of the FFM, which is the strongest predictor of EE (2), explaining more than 70% of the variance in 24h-EE alone (1). BAT may play a role in mediating cold-induced thermogenesis in humans (26). Thus, if the cellular mechanisms described in WAT are representative of those occurring in skeletal muscle, as indicated by the observed influence of muscle sphingolipids on mitochondrial respiration (19), or other organs this could be the underlying mechanism which affects EE. Consistent associations between specific sphingomyelin compounds in muscle and adipose tissue with EE provide further evidence that in these body compartments the same cellular mechanisms could underlie the implication of candidate sphingolipids in EE modulation (supplemental table 3 and 4). Since relatively lower EE predicts weight gain in this population, the observation that some specific sphingomyelins could also be independent predictors of weight change may support a regulatory role of skeletal muscle sphingomyelins in EE regulation (3, 4, 19). Whether sphingomyelins may affect weight development primarily via EE regulation or via energy intake is unclear, since a link between sphingolipids as regulators of food intake has also been described in Drosophila (27).
PCA reduced data dimensionality and identified groups of skeletal muscle sphingolipids by considering their relative relationship to each other (supplemental table 1 and 2, supplemental figure 1 and 2). The C-factor correlated with residual 24h-EE and tended to correlate with weight change (figure 1E and F). SM-factor 1 was positively associated with weight change (figure 1B). For SM-factor 1, a negative association with SMR could be shown, however, it remained not significant (figure 1A). The lack of correlation between SM-factor 1 and an EE measurement could be attributed to the heterogeneous associations of its constituents with EE measures. Also, food intake, the alternate driving force of weight change, can be influenced by sphingolipids. SM-factor 2 correlated with SMR, but not with weight change (figure 1C and D).
With respect to their individual constituents, sphingolipid factors unmasked candidate lipids for further metabolic exploration. In line with the associations of the factors as stated above, muscle ceramides generally tended to correlate with residual 24h-EE, whereas some sphingomyelins showed strong associations mostly with SMR (figure 2). Interestingly, SM-factor 1 comprised all sphingomyelins, which independently associated with weight change.
Correlations between SMR and adipose SM18:1/23:0, SM18:1/23:1, SM18:0/24:1, and SM18:1/24:2 levels were largely consistent with associations observed for these sphingomyelins in muscle and SMR or RMR (supplemental table 4). Adipose SM18:1/23:0 accounted for 7% of the additional variance of SMR. As WAT exhibits a low oxidative capacity (28), the amount of explained variance in EE most likely cannot be attributed to a modulation of adipocyte thermogenesis. Yet, this aspect is addressed by the demonstration of the possible correlation of lipid effectors of EE across tissues (supplemental table 3–5). Studies have proposed, that de novo synthesis of sphingomyelins is reflective of nutrient oversupply in metabolically active organs, where deposition of sphingolipids has a direct effect on their metabolic activity by impairing their cellular function (15, 29, 30). Moreover, a potential influence of specific adipose sphingomyelins on EE could be explained by their role in endocrine signaling: In obese, toxic fat metabolites from adipose tissue exert a pathologic effect on distant tissues (31). In rodents, it was shown that the distant effect of ceramides on the central nervous system led to altered EE via inhibition of the sympathoadrenal effect on BAT (20). Associations between sphingomyelin plasma and adipose concentrations may be indicative of such lipid messaging behavior. The mechanism of sphingolipid EE regulation across tissues remains elusive. However, the association of pooled sphingomyelin concentrations for adipose and muscle tissue with EE and commonalities across different body compartments might imply that sphingolipids of different body compartments act in concert to modulate EE.
Some sphingolipids within skeletal muscle tissue did not correlate with EE but associated with weight gain (figure 1). This could be explained by the general heterogeneity of association with residual EE (figure 2) and the ability of sphingomyelins and ceramides to interconvert (32), yielding alternate sphingolipid variants (10, 11), which may ultimately affect thermogenesis. SM18:1/23:0 correlated negatively with carbohydrate oxidation and 24h-RQ. As both higher concentrations of SM18:1/23:0 and higher 24h-RQ have been associated with weight gain (33), it may be that the mechanism underlying higher 24h-RQ is independent of any effect of sphingolipids. Also, sphingolipid associations with AFT, RMR and SMR, as well as 24h-EE support the assumption, that a thermogenic effect of these lipids may be independent of physical activity.
Of note, adjustment of weight change for initial weight was performed despite lack of correlation between annual weight change and initial weight (34). However, the results were consistent if absolute weight change per year was used (figures 1 and 3).
Differences in association patterns observed for certain muscle sphingomyelins comparing RMR and SMR may need to be interpreted in the light of the distinct metabolic states these measures represent, and the methods used for their assessment. RMR is measured during an awake motionless state for 40 minutes and so reflects the cost of arousal (2). SMR, however, as assessed in the metabolic chamber, is defined as the EE during night hours with minimal SPA. During the sleeping state cardiovascular activity and body temperature are altered compared with the awake alert state (35). Given that skeletal muscle accounts for a large amount of variance in SMR and RMR (~ 20%) (36, 37), it is in line with this that multiple sphingolipid moieties in skeletal muscle did correlate with SMR. Although the associations of the identified sphingolipids are in the same direction, the lack of agreement between SMR and RMR may be due to the physiologic difference in what is measured as well as the methodology used.
Notably, here reported associations of several skeletal muscle or adipose sphingolipids with EE measures cannot be translated to all sphingolipids. Present analyses were exploratory in nature and present preliminary data. The role of sphingolipids as a class and the implication of here presented candidate sphingolipids in energy balance regulation needs to be further elucidated in future studies.
Limitations to our study are given by the small sample size (particularly for the weight change analysis) from a population not representative of a more heterogeneous heritage. Although our conclusions are restricted to the studied population, results drawn from studies based on volunteers of Southwestern Native American heritage have been confirmed in other populations (38). For muscle sphingomyelins, two significant factors emerged from PCA, together explaining more than ⅔ (= 68%) of the sphingomyelins’ global variance. A possible limitation of PCA to analyze present data (subject-to-variable ratio = 1.75) was therefore overcome by the strong pairwise correlations among sphingomyelins. To minimize the issue of multiple comparisons and to identify biologically true associations, only those sphingolipids were introduced into correlation analyses with EE measurements which were considered strong (r > 0.6) determinants of PCs associated with EE or weight gain. Also, for candidate sphingolipids, the involvement in EE regulation was questioned by investigating a possible effect on weight gain as an additional metabolic factor. Furthermore, results in skeletal muscle were replicated in adipose tissue. As our analysis was designed to generate hypotheses about the association of lipid signaling molecules and EE, alpha-level was set to 0.05. Future studies clearly need to replicate associations between sphingomyelins and weight change in a larger population. Diet and physical activity may have been mitigating factors in weight change but this information was not collected in these studies.
In conclusion, we provide preliminary evidence that skeletal muscle and subcutaneous adipose tissue concentrations of specific ceramides and sphingomyelins explain a large amount of the unexplained variance of EE in Native Americans of Southwestern heritage. Accordingly, we found that certain muscle sphingomyelins may predict weight change in this study population. Our explorative study sets the stage for new intervention studies to further explore human EE beyond the known anthropometric determinants, and the role of lipid mediators, specifically sphingolipids, in human energy balance regulation.
Supplementary Material
Acknowledgments
We thank all volunteers whose participation in clinical trials contributed to this study.
Grants/fellowships supporting the writing of the paper: NIH Intramural Research Fund, Berlin Institute of Health (BIH) Charité Clinician Scientist Program
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Information
Supplementary information is available at the International Journal of Obesity’s website.
References
- 1.Weyer C, Snitker S, Rising R, Bogardus C, Ravussin E. Determinants of energy expenditure and fuel utilization in man: effects of body composition, age, sex, ethnicity and glucose tolerance in 916 subjects. International Journal of Obesity & Related Metabolic Disorders. 1999;23(7):715–22. doi: 10.1038/sj.ijo.0800910. [DOI] [PubMed] [Google Scholar]
- 2.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. Journal of Clinical Investigation. 1986;78(6):1568. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravussin E, Bogardus C. A brief overview of human energy metabolism and its relationship to essential obesity. The American journal of clinical nutrition. 1992;55(1):242S–5S. doi: 10.1093/ajcn/55.1.242s. [DOI] [PubMed] [Google Scholar]
- 4.Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. The Journal of Clinical Endocrinology & Metabolism. 2013;98(4):E703–E7. doi: 10.1210/jc.2012-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinhardt M, Thearle MS, Ibrahim M, Hohenadel MG, Bogardus C, Krakoff J, et al. A human thrifty phenotype associated with less weight loss during caloric restriction. Diabetes. 2015;64(8):2859–67. doi: 10.2337/db14-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, et al. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10(5):524–9. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 7.Krakoff J, Ma L, Kobes S, Knowler WC, Hanson RL, Bogardus C, et al. Lower metabolic rate in individuals heterozygous for either a frameshift or a functional missense MC4R variant. Diabetes. 2008;57(12):3267–72. doi: 10.2337/db08-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. Jama. 2012;307(5):483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michel V, Bakovic M. Lipid rafts in health and disease. Biology of the Cell. 2007;99(3):129–40. doi: 10.1042/BC20060051. [DOI] [PubMed] [Google Scholar]
- 10.Park T-S, Panek RL, Mueller SB, Hanselman JC, Rosebury WS, Robertson AW, et al. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E–knockout mice. Circulation. 2004;110(22):3465–71. doi: 10.1161/01.CIR.0000148370.60535.22. [DOI] [PubMed] [Google Scholar]
- 11.Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, et al. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. Journal of Biological Chemistry. 2005;280(11):10284–9. doi: 10.1074/jbc.M412348200. [DOI] [PubMed] [Google Scholar]
- 12.Milhas D, Clarke CJ, Hannun YA. Sphingomyelin metabolism at the plasma membrane: implications for bioactive sphingolipids. FEBS letters. 2010;584(9):1887–94. doi: 10.1016/j.febslet.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillard B, Thomas J, Nell L, Marcus D. Antibodies against ganglioside GT3 in the sera of patients with type I diabetes mellitus. The Journal of Immunology. 1989;142(11):3826–32. [PubMed] [Google Scholar]
- 14.Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell metabolism. 2013;17(5):790–7. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, et al. A critical role for PPARα-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proceedings of the National Academy of Sciences. 2003;100(3):1226–31. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. American Journal of Physiology-Endocrinology and Metabolism. 2009;297(1):E211–E24. doi: 10.1152/ajpendo.91014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, et al. Obesity-induced CerS6-dependent C 16: 0 ceramide production promotes weight gain and glucose intolerance. Cell metabolism. 2014;20(4):678–86. doi: 10.1016/j.cmet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Vidal-Puig A, Solanes G, Grujic D, Flier JS, Lowell BB. UCP3: an uncoupling protein homologue expressed preferentially and abundantly in skeletal muscle and brown adipose tissue. Biochemical and biophysical research communications. 1997;235(1):79–82. doi: 10.1006/bbrc.1997.6740. [DOI] [PubMed] [Google Scholar]
- 19.Smith ME, Tippetts TS, Brassfield ES, Tucker BJ, Ockey A, Swensen AC, et al. Mitochondrial fission mediates ceramide-induced metabolic disruption in skeletal muscle. Biochemical Journal. 2013;456(3):427–39. doi: 10.1042/BJ20130807. [DOI] [PubMed] [Google Scholar]
- 20.Contreras C, González-García I, Martínez-Sánchez N, Seoane-Collazo P, Jacas J, Morgan DA, et al. Central ceramide-induced hypothalamic lipotoxicity and ER stress regulate energy balance. Cell reports. 2014;9(1):366–77. doi: 10.1016/j.celrep.2014.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genuth S, Alberti K, Bennett P, Buse J, DeFronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes care. 2003;26(11):3160–8. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 22.Realini N, Solorzano C, Pagliuca C, Pizzirani D, Armirotti A, Luciani R, et al. Discovery of highly potent acid ceramidase inhibitors with in vitro tumor chemosensitizing activity. Scientific reports. 2013;3:1035. doi: 10.1038/srep01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basit A, Piomelli D, Armirotti A. Rapid evaluation of 25 key sphingolipids and phosphosphingolipids in human plasma by LC-MS/MS. Analytical and bioanalytical chemistry. 2015;407(17):5189–98. doi: 10.1007/s00216-015-8585-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knowler WC, Pettitt DJ, Saad MF, Charles MA, Nelson RG, Howard BV, et al. Obesity in the Pima Indians: its magnitude and relationship with diabetes. The American journal of clinical nutrition. 1991;53(6):1543S–51S. doi: 10.1093/ajcn/53.6.1543S. [DOI] [PubMed] [Google Scholar]
- 25.Schutz Y, Ravussin E, Diethelm R, Jequier E. Spontaneous physical activity measured by radar in obese and control subject studied in a respiration chamber. International journal of obesity. 1981;6(1):23–8. [PubMed] [Google Scholar]
- 26.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. New England Journal of Medicine. 2009;360(15):1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 27.Walls SM, Jr, Attle SJ, Brulte GB, Walls ML, Finley KD, Chatfield DA, et al. Identification of sphingolipid metabolites that induce obesity via misregulation of appetite, caloric intake and fat storage in Drosophila. PLoS Genet. 2013;9(12):e1003970. doi: 10.1371/journal.pgen.1003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kopecký J, Rossmeisl M, Flachs P, Bardova K, Brauner P. Mitochondrial uncoupling and lipid metabolism in adipocytes. Biochemical Society Transactions. 2001;29(6):791–7. doi: 10.1042/bst0290791. [DOI] [PubMed] [Google Scholar]
- 29.Ibrahim SH, Kohli R, Gores GJ. Mechanisms of lipotoxicity in NAFLD and clinical implications. Journal of pediatric gastroenterology and nutrition. 2011;53(2):131–40. doi: 10.1097/MPG.0b013e31822578db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi S, Snider AJ. Sphingolipids in High Fat Diet and Obesity-Related Diseases. Mediators of inflammation. 2015;2015 doi: 10.1155/2015/520618. Published online 2015 Nov 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome—an allostatic perspective. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2010;1801(3):338–49. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Mitsutake S, Zama K, Yokota H, Yoshida T, Tanaka M, Mitsui M, et al. Dynamic modification of sphingomyelin in lipid microdomains controls development of obesity, fatty liver, and type 2 diabetes. Journal of Biological Chemistry. 2011;286(32):28544–55. doi: 10.1074/jbc.M111.255646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gluck ME, Venti CA, Salbe AD, Votruba SB, Krakoff J. Higher 24‐h Respiratory Quotient and Higher Spontaneous Physical Activity in Nighttime Eaters. Obesity. 2011;19(2):319–23. doi: 10.1038/oby.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piaggi P, Krakoff J, Bogardus C, Thearle MS. Lower “awake and fed thermogenesis” predicts future weight gain in subjects with abdominal adiposity. Diabetes. 2013;62(12):4043–51. doi: 10.2337/db13-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burgess HJ, Trinder J, Kim Y, Luke D. Sleep and circadian influences on cardiac autonomic nervous system activity. American Journal of Physiology-Heart and Circulatory Physiology. 1997;273(4):H1761–H8. doi: 10.1152/ajpheart.1997.273.4.H1761. [DOI] [PubMed] [Google Scholar]
- 36.Rolfe DF, Newman JM, Buckingham JA, Clark MG, Brand MD. Contribution of mitochondrial proton leak to respiration rate in working skeletal muscle and liver and to SMR. American Journal of Physiology-Cell Physiology. 1999;276(3):C692–C9. doi: 10.1152/ajpcell.1999.276.3.C692. [DOI] [PubMed] [Google Scholar]
- 37.Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. Journal of Clinical Investigation. 1990;86(5):1423. doi: 10.1172/JCI114857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stefan N, Stumvoll M, Weyer C, Bogardus C, Tataranni P, Pratley R. Exaggerated insulin secretion in Pima Indians and African‐Americans but higher insulin resistance in Pima Indians compared to African‐Americans and Caucasians. Diabetic medicine. 2004;21(10):1090–5. doi: 10.1111/j.1464-5491.2004.01290.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


