Figure 3.
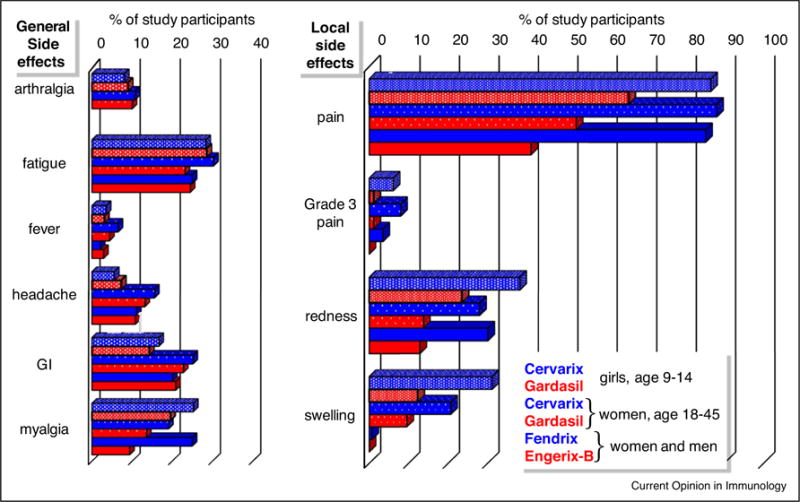
Inflammatory side effects of vaccines adjuvanted with alum + MPL or alum. The percentages of participants who experienced general or local adverse events after vaccine dose 1 in the clinical studies described in Figure 2 are shown. Blue and red bars: instances of adverse events after intramuscular injection of vaccines containing alum + MPL (Cervarix and Fendrix) or alum alone (Gardasil and Engerix-B), respectively. Instances of arthralgia were not recorded after administration of Fendrix or Engerix-B. For each cluster shown, bars from top-to-bottom correspond to adverse events after injection of Cervarix versus Gardasil in N = 716 girls age 9–14 from France, Hong Kong, Singapore and Sweden; Cervarix versus Gardasil in N = 249 women age 18–26 from the US; and Fendrix versus Engerix-B in N = 282 adult women and men age 18–45 from Belgium and Germany. Data from [53,55,57]; N indicates all participants enrolled in each study who received vaccine dose 1 regardless of pre-immune status or completion of the immunization series according-to-protocol.
