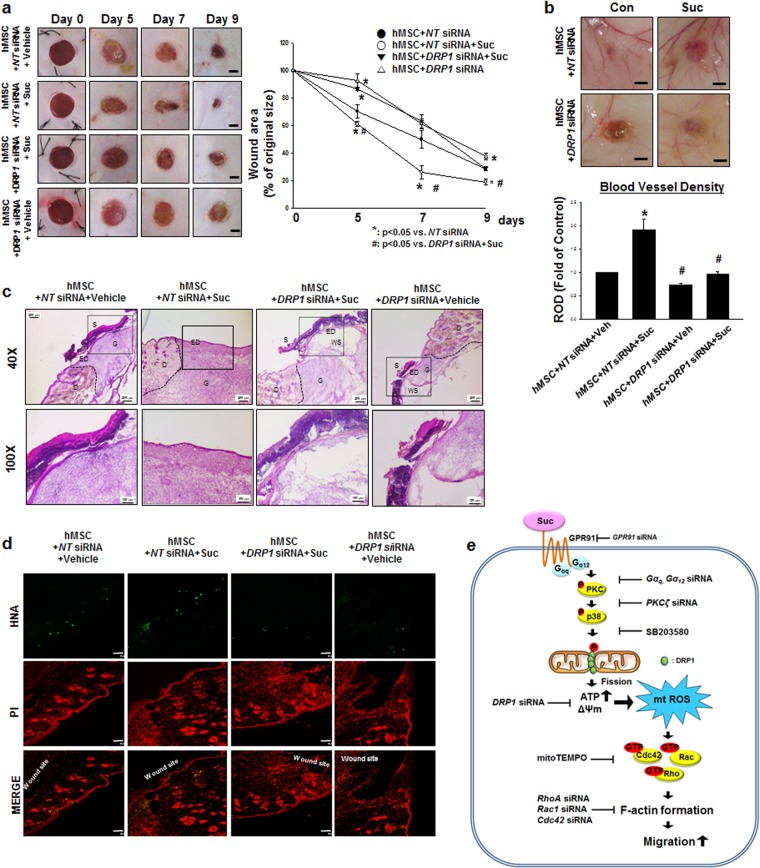
Figure 7.
The role of DRP1 on skin wound healing in vivo. (a) Representative gross images of skin wound healing at day 0, 5, 7, and 9 are shown. Mouse skin wounds were surgically made by 6-mm-diameter biopsy punch and treated with hMSC + NT siRNA + vehicle, hMSC + NT siRNA + succinate, hMSC + DRP1 siRNA + succinate or hMSC + DRP1 siRNA + vehicle, respectively. (left panel). Wound healing was determined by assessing the percentage of wound closures relative to original wound size. Data represent mean ± SEM. n = 6, *p < 0.05 versus NT siRNA, #p < 0.05 versus DRP1 siRNA + succinate (right panel), Scale bars = 2 mm. (b) The quantified vascularity wound area at day 9 was shown. Data represent mean ± SEM. n = 6, *p < 0.05 versus hMSC with NT siRNA, #p < 0.05 versus hMSC with NT siRNA and succinate, Scale bars = 2 mm. (c) Representative wound tissues stained with H&E at day 9 were shown. n = 6, Scale bars = 200 μm or 100 μm, magnification; ×40 or ×100, respectively. (d) Engraftment of hMSCs on wound at day 9 was observed by using confocal microscopy. Human nuclear antigen (HNA, green) was used for hMSC nuclear staining. Propidium iodide (PI, red) was used for nuclear counter staining, Scale bars = 100 μm, magnification; ×100. (e) Hypothetical model for the effect of succinate on hMSC migration through mitochondrial fission. Succinate induced PKC phosphorylation through GPR91 activation. Then PKC activated p38 MAPKs and subsequently DRP1 phosphorylation. DRP1 phosphorylation caused mitochondrial fission and finally enhancement of mitochondrial ATP production and mitochondrial membrane potential. In result of increased mitochondrial functions, mtROS was produced and induced Rho GTPases activation. Finally, activated Rho GTPases caused F-actin formation to promote hMSC motility. Abbreviations: S, scab; D, dermis; ED, epidermis; G, granular tissue; WS, wound site; ΔΨm, mitochondrial membrane potential.