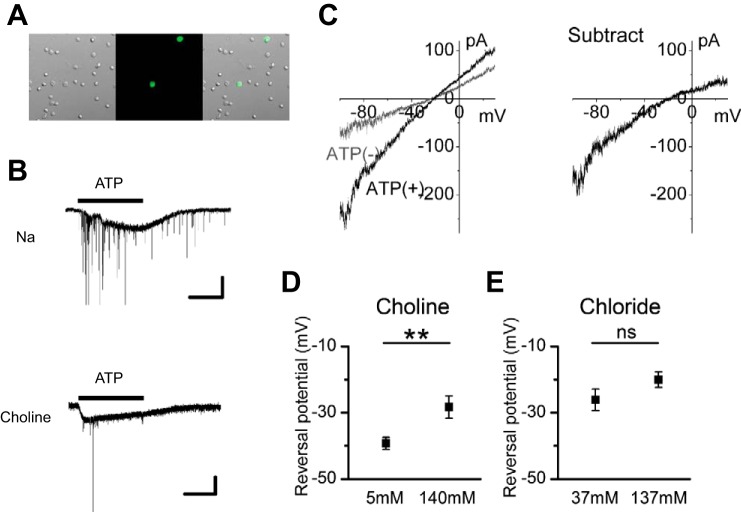
Fig. 2.
The ionic mechanism of the ATP-induced inward current. A: photomicrographs showing GFP-positive CAs in dissociated retinal preparations. Left, a differential interference image; middle, a fluorescence image; right, a merged image. B: responses to 100 μM ATP in Na-rich solution (no. 2-1; top) and in choline-rich solution (no. 2-2; bottom). The large, slow inward current is the P2 purinoceptor (including P2X2 purinoceptor)-mediated cation current. Inhibitory postsynaptic currents superimposed on the P2 purinoceptor-mediated cation current are a result of activation of P2 purinoceptors on the presynaptic terminals of CAs (Kaneda et al. 2008). The timing of ATP application is indicated by the horizontal bar above the current trace. Experiments were conducted in dissociated cell preparations. C: 2 I-V curves obtained by ramp pulse before [ATP(−); gray line] and during [ATP(+); black line] application of 100 μM ATP (left) and subtracted I-V curves (right). The extracellular solution is 140 choline (no. 3-1). D: shift of the reversal potential of the ATP-induced inward currents by choline substitution. Values are means ± SE; n = 23 cells from 6 mice for 140 choline solution (no. 3-1); n = 15 cells from 4 mice for 5 choline solution (no. 3-2). E: shift of the reversal potential of the ATP-induced inward currents by extracellular Cl− substitution. Values are means ± SE; n = 12 cells from 8 mice for 137 Cl solution (no. 4-1); n = 8 cells from 4 mice for 37 Cl solution (no. 4-2). **P < 0.01; ns, not significant (unpaired t-test). Intracellular solutions were KCl (no. In-1) in B and E and CsCl (no. In-2) in C and D.