FIG 2.
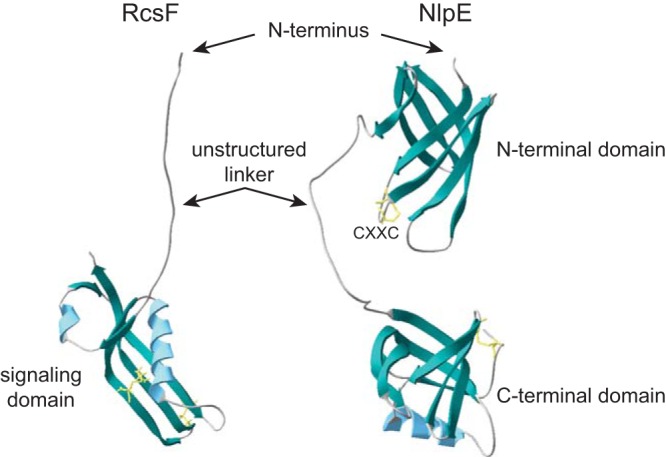
Structures of RcsF and NlpE. Ribbon representations of the protein structures of RcsF (left, PDB 2L8Y [44]) and NlpE (right, PDB 2Z4H [53]). Predicted β-sheets are in green, α-helices are in blue, and disulfide bonds are in yellow. The N terminus of the proteins (lacking the lipobox and signal peptide) and domain composition are indicated. RcsF harbors an unstructured linker region at the N terminus and a globular domain, which is sufficient for Rcs activation. Type I NlpE homologs have both the N-terminal and the C-terminal domains, separated by a region that is predicted to be unstructured and flexible, whereas type II homologs only carry the N-terminal domain. A highly conserved CXXC motif is present in the N-terminal domain of NlpE.
