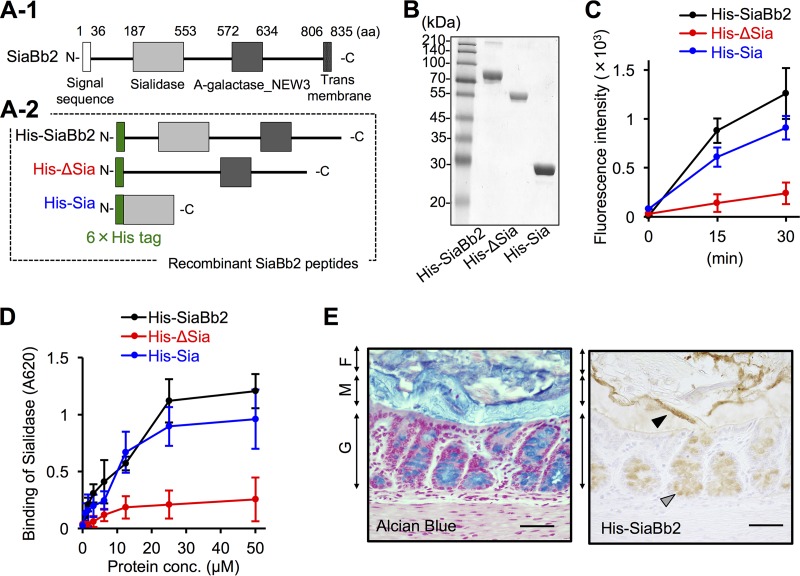
FIG 3 .
Binding properties of recombinant sialidase peptides. (A-1) Schematic representation of the primary structure of SiaBb2 from B. bifidum ATCC 15696. Amino acid numbering starts at the presumed initiation codon. Sialidase and alpha-galactosidase NEW3 (A-galactosidase_NEW3) domains are depicted as light and dark gray boxes, respectively. The white box at the N terminus and the gridded box at the C terminus indicate a signal peptide and membrane anchor, respectively. (A-2) Schematic representation of SiaBb2-truncated peptides expressed as fusions to 6×His tag at the N terminus (green boxes). (B) Purified SiaBb2-truncated peptides (10 μg per lane) from B. bifidum ATCC 15696 were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and visualized by staining with Coomassie brilliant blue. The molecular mass standard is shown on the left. (C) Sialidase activity of SiaBb2-truncated proteins. Recombinant proteins (50 μM) were incubated with 4-MU-Neu5Ac, and fluorescence was recorded for 30 min. Data are presented as means ± standard deviations (n = 5). (D) Binding of SiaBb2-truncated peptides to PCM, as determined by enzyme-linked immunosorbent assay. PCM-coated wells were incubated with 0.025 to 50 μM recombinant peptide. Binding was detected with an anti-His antibody. Data are presented as means ± standard deviations (n = 3). (E) His-SiaBb2 binding to murine colonic tissue sections. Total glycoconjugates were detected by alcian blue staining. Biotinylated His-SiaBb2 specifically reacted with the mucosal surface at the border between the mucus layer and fecal material (black arrowhead) and with goblet cells (gray arrowhead). F, fecal material; M, mucus layer; G, goblet cells. Bars, 200 μm.