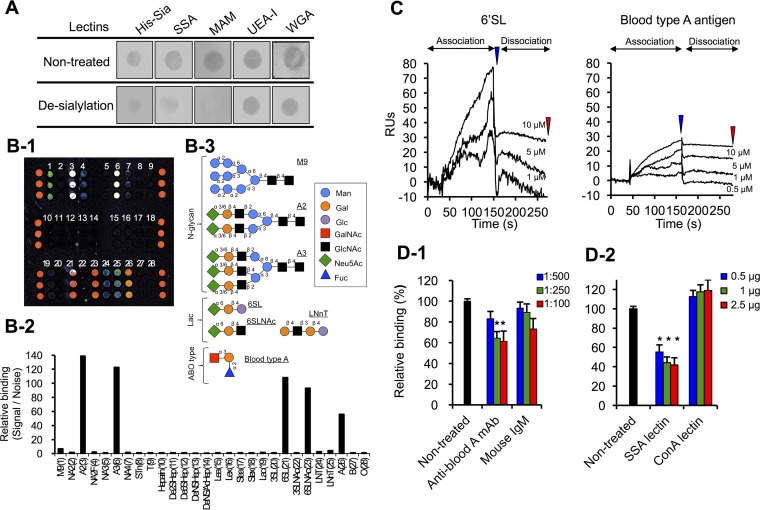
FIG 4 .
SiaBb2 binds to sialyl-(α2,6)Gal and BgA antigen. (A) Effects of desialylation with mild acid hydrolysis on binding of His-Sia and lectins to PCM. PCMs were immobilized on a nitrocellulose membrane. Binding of biotinylated His-Sia or lectins was detected with horseradish peroxidase-labeled streptavidin. SSA, S. sieboldiana; MAM, M. amurensis; UEA-I, U. europaeus; WGA, wheat germ agglutinin. (B) Glycoarray analysis. (B-1) Fluorescence intensity of His-Sia on the glycoarray plate measured with a fluorescence scanner. (B-2) Fluorescence signals presented as a histogram of signal/noise ratios calculated from three independent spots (fluorescence intensity of binding His-Sia signal divided by background noise); each bar represents a carbohydrate. (B-3) Schematic illustration of representative carbohydrate structures described in the study. (C) SPR sensorgrams of His-Sia interaction with 6'SL and BgA trisaccharide. Carbohydrates were added to a CM5 sensor chip with immobilized ligand His-Sia. Analyte concentrations (from top to bottom) are 10, 5, 1, and 0.5 μM. Blue and red arrowheads indicate the end of association and end of dissociation, respectively. (D) Inhibition of His-Sia binding to immobilized PCM by anti-BgA antibody (D-1) or sialyl-(α2,6)Gal-binding S. sieboldiana (SSA) lectin and mannose-binding ConA lectin (D-2). Binding of His-Sia to PCM, as determined by ELISA. Data represent means ± standard deviations (n = 5). Significant differences were determined by analysis of variance with a Tukey test. *, P < 0.05 versus His-Sia peptides preincubated without antibody (untreated) or lectins.