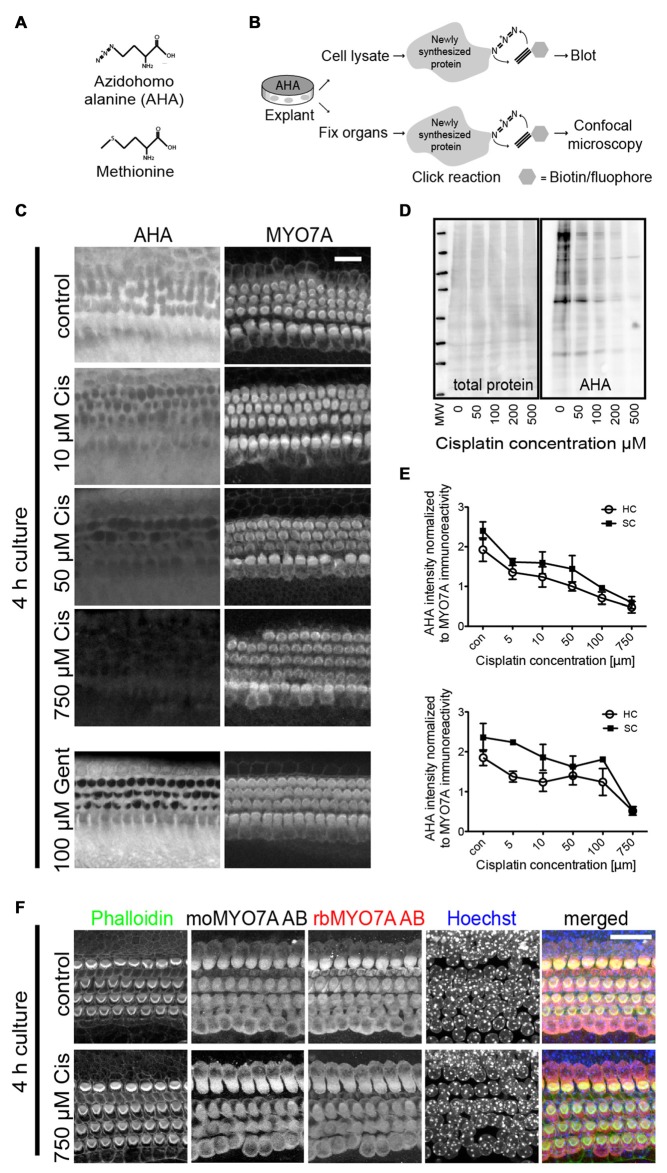
Figure 1.
Bioorthogonal noncanonical amino acid tagging (BONCAT) to study protein synthesis within sensory hair cells of mouse explant cultures. (A) Chemical structure of methionine and its utilized analog, azidohomoalanine (AHA). (B) Schematic of the BONCAT technique using either cell lysates for immunoblot or fixed organs for fluorescence microscopy. (C) AHA-biotin immunoreactivity demonstrates dose-dependent inhibition of protein synthesis on a cell-by-cell basis after treatment with cisplatin. P3–4 organ of Corti explants were cultured in growth medium containing AHA, in the presence of varying cisplatin concentrations. After 4 h, prior to the onset of cisplatin-induced cell death or changes in Myosin 7a (MYO7A) levels, explants were fixed and processed for click-chemistry reaction and imaged using confocal microscopy. Protein synthesis is inhibited in both hair cells and supporting cells. Gentamicin induced inhibition of cellular protein synthesis shown to involve only hair cells (bottom panels). Scale bar 20 μm. (D) Immunoblot showing a decrease in cellular protein synthesis within organ of Corti explant lysates. AHA-biotin was detected with streptavidin (SA)-horseradish peroxidase (HRP). (E) Quantification of AHA uptake relative to immunoreactivity of MYO7A within mouse cochleae and utricles (n = 4). There is a marked dose-dependent reduction in AHA uptake in both sensory hair cells (HC) and supporting cells (SC). Error bars indicate SEM (standard error of the mean). (F) MYO7A immunoreactivity and nuclear morphology is not affected by short exposure (4 h) to high concentrations of cisplatin, demonstrating the appropriateness of using MYO7A staining to normalize the AHA signal.