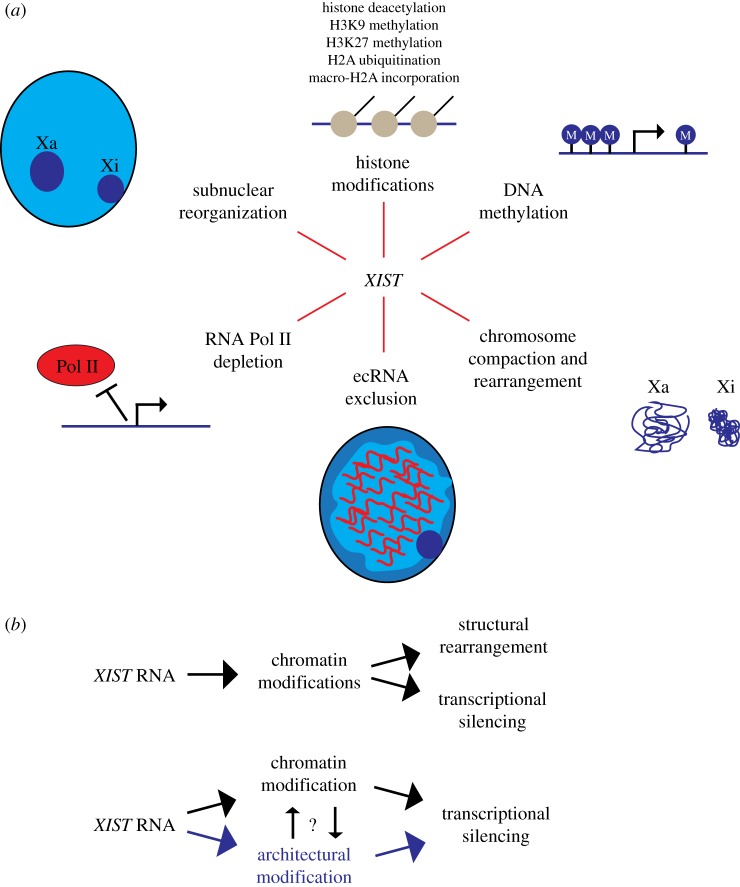
Figure 1.
Events in X-chromosome inactivation downstream of XIST RNA expression. (a) Induction and spreading of the XIST RNA on the X chromosome directly or indirectly triggers a number of changes leading to robust transcriptional silencing. The timing and interplay between these events is only beginning to be understood. XIST is thought to directly bind and recruit one or more histone modifying enzymes which introduce histone deacetylation, methylation and ubiquitination of H2A, leading to other downstream modifications, including incorporation of histone variant macro-H2A, and CpG methylation, as well as other changes to create an environment that represses transcription. This series of numerous histone modifications coincides with large-scale changes in nuclear organization, including exclusion of repeat-rich euchromatin-associated RNAs (ecRNAs) and Pol II, chromatin compaction (visible DNA condensation), topological rearrangement of the chromosome territory, and movement of Xi to the peripheral heterochromatin compartment (or nucleolus). Which events occur first and how these changes synergize to transcriptionally repress the whole chromosome remain to be fully established. (b) Current models emphasize the function of XIST RNA in directly recruiting histone modifying enzymes which may be responsible for transcriptional silencing and cytological-scale changes in chromosome condensation and structure (upper pathway). An alternative model is outlined in the lower pathway whereby XIST RNA may also directly interact and impact architectural elements of non-chromatin structure (highlighted in purple), which modifies the higher-order chromatin folding to promote chromosome condensation, movement to peripheral heterochromatin, or other overall structural changes. Thus, by interacting with a distinct set of architectural proteins (such as SAF-A) XIST RNA could act in parallel with chromatin modifiers, and collectively these changes may induce both gene silencing and a highly stable heterochromatic structure.