Abstract
Shiba dogs are predisposed to chronic enteropathy (CE) and have poorer prognosis than other dog breeds. The objective of this study was to investigate the significance of polymerase chain reaction for antigen receptor rearrangement (PARR) results on clinical findings and prognosis of Shiba dogs with CE. We retrospectively collected data on 22 Shiba dogs diagnosed as having CE. Fifty-nine percent of the dogs had clonality-positive results on PARR analysis. Furthermore, on histopathology, epitheliotropic behavior of small lymphocytes of the intestinal mucosa was observed significantly more frequently in dogs with clonal rearrangement of antigen receptor genes (P=0.027). The median overall survival time of clonality-positive dogs was 48 days (range, 4–239 days), compared to 271 days (range, 45–1,316+ days) in clonality-negative dogs. The median overall survival time of epitheliotropism-positive dogs was 76 days (range, 30–349 days) compared to 239 days (range, 4–1,316+ days) for epitheliotropism-negative dogs. Statistical analysis revealed that the clonality-positive result was associated with significantly shorter survival time (P=0.036). In contrast, presence or absence of epitheliotropism had no statistically significant effect on survival time (P=0.223). These cases might appropriately be diagnosed as small T-cell intestinal lymphoma; there are some common clinical and pathogenic features with human enteropathy-associated T-cell lymphoma type 2. The pathogenesis and poor prognosis for Shiba dogs with CE seem to be associated with this type of lymphoma, although further investigation is warranted.
Keywords: chronic enteropathy, dog, lymphoma, polymerase chain reaction for antigen receptor rearrangements, Shiba dogs
Chronic enteropathy (CE) is common in dogs and is characterized by persistent or recurrent gastrointestinal signs, including diarrhea, vomiting and weight loss. Chronic enteropathy is categorized into three groups; antibiotics-responsive diarrhea (ARD), food-responsive diarrhea (FRD) and inflammatory bowel disease (IBD) [1, 12]. The prognosis is favorable, if the condition is treated properly. For example, the three-year survival rate for dogs with FRD is 97% and is 57% for dogs with IBD [2]. The etiology of CE is unknown, although breed-specific susceptibility has been suggested [16].
We have previously demonstrated that the Shiba dogs are predisposed to CE and have a significantly poorer outcome, when compared to other dog breeds [22]. The median overall survival time for Shiba dogs with CE is 74 days, with a 6-month, 1-year and 3-year survival of 46, 31 and 0%, respectively. Severe inflammatory lesions, consisting of small lymphocytes and plasma cells, were found in the duodenum of 75% of the Shiba dogs with CE. These severe changes in the duodenum may represent a characteristic feature of CE in Shiba dogs. Several reports describe refractory CE and decreased survival in Shiba dogs [24, 25]. However, the reason for the poor prognosis for Shiba dogs with CE has not been elucidated.
Polymerase chain reaction (PCR) for antigen receptor rearrangements (PARR) analysis has been examined as a diagnostic aid for lymphoid neoplasms [3, 17]. This method detects a clonal population of lymphocytes, by amplifying the T-cell receptor gamma-chain gene (TCRγ) and the immunoglobulin heavy chain gene (IgH). This technique has the ability to differentiate reactive lymphoid cell proliferation from neoplastic lymphocytes and can be used to distinguish inflammation from lymphoma. When considering gastrointestinal lymphoma, however, results from intestinal biopsy PARR analyses should not be interpreted independent of histopathology. Neoplastic lymphoid cells are often associated with reactive or inflammatory lymphocytes, a condition that decreases the accuracy of the test. The reported sensitivity of intestinal biopsy samples to diagnose canine alimentary lymphoma is between 66.7 and 76%, much lower than the reported sensitivity of >90% for detecting clonal lymphoid populations [5, 10, 11, 13, 23]. The relatively low sensitivity suggests that lack of monoclonality on PARR analysis cannot exclude the diagnosis of lymphoma. Furthermore, clonality was observed in 51% of dogs diagnosed with CE, suggesting low specificity of the test for this condition [13]. Clonal rearrangement of lymphocyte antigen receptor may be a negative prognostic factor in CE [15, 20]. From those results, there seems to be a room for evaluation of the significance and utility of PARR analysis in CE and gastrointestinal lymphoma.
The purpose of this study was to investigate the significance of PARR analysis on clinical findings and prognosis of Shiba dogs with CE, and to determine if clonal antigen receptor rearrangements are associated with the pathogenesis of CE in this dog breed.
MATERIALS AND METHODS
Case selection
Medical records of Shiba dogs diagnosed with CE in the Veterinary Medical Center of the University of Tokyo, between 2007 and 2012, were collected and reviewed retrospectively. All dogs included in the study met all criteria for diagnosis of CE. The following criteria were used: having chronic (>3 weeks) gastrointestinal signs, such as vomiting, diarrhea, anorexia and weight loss; exclusion of other causes of chronic gastrointestinal signs by thorough diagnostic evaluation; and histopathological evidence of lymphocytic or lymphoplasmacytic infiltration in the gastrointestinal tract. Cases with gastrointestinal masses or proliferation of large lymphoid cells, suggesting large cell lymphoma, were excluded. Since small cell gastrointestinal lymphoma was not clearly defined in dogs, we included dogs with small lymphocytes infiltration all together. Food-responsive enteropathy and antibiotics-responsive enteropathy were not exhaustively ruled out before endoscopic examination, because of rapid worsening of disease in almost all dogs. Cases without genomic DNA extracted from the biopsy sample were excluded.
Histopathology
Endoscopy of the upper gastrointestinal tract was performed in all dogs, and lower gastrointestinal tract endoscopy was also done when needed. Six or more samples were obtained per site (stomach, duodenum, ileum and colon). Samples were routinely fixed in 10% neutral buffered formalin, embedded in paraffin, cut into sections, and stained with hematoxylin and eosin (HE). Histopathological severity was objectively classified into four grades, (normal, mild, moderate and severe), based on the degree of inflammatory cell infiltration and structural changes (i.e., stunting and fusion of villi, lacteal dilation, epithelial injury, mucosal fibrosis and crypt distension) in the gastrointestinal mucosa. Intraepithelial infiltration by small lymphocytes was evaluated objectively according to the number and density of intraepithelial lymphocytes. Marked and dense infiltration of small lymphocytes in the epithelium was judged as epitheliotropism-positive. None to only mild increase in intraepithelial lymphocytes was classified as epitheliotropism-negative.
PCR for antigen receptor rearrangements (PARR)
To detect the presence of clonal rearrangement in the IgH or TCRγ genes, genomic DNA was extracted from biopsy specimens and submitted for PARR clonality assay [10]. Specimens from the most severely affected sites identified on histopathology were used for PARR analysis. In some dogs, samples from different gastrointestinal sites were mixed in the same one tube and analyzed as a whole. Detection of a single sharp band was interpreted as a clonality-positive result.
Data analysis
Based on the PARR results, cases were divided into two groups: clonality-positive and clonality-negative. Signalment, clinical signs and duration of the history, laboratory results, histopathology and outcomes were recorded and compared between the two groups. Clinical signs were evaluated using two previously described clinical scoring indices: the canine chronic enteropathy clinical activity index (CIBDAI) and the canine chronic enteropathy clinical activity index (CCECAI) [2, 14]. The following laboratory parameters were evaluated: packed cell volume (PCV), total white blood cells (WBC), platelet count (Plt), total protein (TP), albumin (Alb), blood urea nitrogen (BUN), creatinine (Cre), alkaline phosphatase (ALP), alanine aminotransferase (ALT), total cholesterol (T-cho), magnesium (Mg), calcium (Ca), sodium (Na), potassium (K), chloride (Cl) and C-reactive protein (CRP).
Statistical analysis
Statistical analysis was performed using commercially available software (StatMate IV version 4.01, ATMS Co., Ltd., Tokyo, Japan). Fisher’s exact test was used to determine associations between categorical variables. Mann-Whitney U tests were used to compare numerical values. Kaplan-Meier survival curves and log-rank testing were used to analyze survival data. A value of P<0.05 was considered statistically significant.
RESULTS
Cases
Twenty-two Shiba dogs were included in this study. Thirteen dogs were male (four were castrated), and nine dogs were female (three were spayed). The median age at the time of diagnosis was 6.0 years (range, 1.3–11.4 years). On PARR analysis, 13 dogs were clonality-positive. Ten dogs demonstrated T-cell monoclonality, one had B-cell monoclonality, and two dogs had both T- and B-cell monoclonality. Nine dogs were clonality-negative. No significant differences in gender or age were seen between groups (Table 1).
Table 1. Signalment, clinical findings and results from blood tests in clonality-positive and clonality-negative Shiba dogs.
| Clonality-positive group (n=13) | Clonality-negative group (n=9) | P | ||
|---|---|---|---|---|
| Signalment | ||||
| Gender, M: MC: F: FS | 6: 3: 3: 1 | 3: 1: 3: 2 | ND | |
| Male: Female ratio | 2.3: 1 | 0.8: 1 | 0.4706 | |
| Age (years) | 6.0 (4.4–8.2) | 6.7 (1.3–11.4) | 0.1009 | |
| Clinical findings | ||||
| Duration of clinical history (months) | 6 (1–48) | 5 (1–24) | 0.7111 | |
| CIBDAI | 8 (2–13) | 7 (4–13) | 0.7113 | |
| CCECAI | 8 (2–17) | 10 (5–14) | 0.4015 | |
| CBC | ||||
| PCV (%) | 40 (29–42) | 37 (23–52) | 0.7372 | |
| WBC (/µl) | 12,200 (2,500–40,500) | 18,400 (12,500–39,400) | 0.0663 | |
| Plt (/µl) | 381,000 (24,000–970,000) | 357,000 (185,000–843,000) | 0.9202 | |
| Serum biochemistry | ||||
| TP (g/dl) | 5.0 (2.8–8.0) | 4.6 (2.6–7.0) | 0.4221 | |
| Alb (g/dl) | 2.0 (1.1–3.4) | 1.9 (1.2–2.5) | 0.482 | |
| BUN (mg/dl) | 13.0 (3.9–37.7) | 14.3 (8.9–27.9) | 0.1814 | |
| Cre (mg/dl) | 0.5 (0.1–1.4) | 0.5 (0.2–1.3) | 0.6129 | |
| ALP (U/l) | 252 (55–2,673) | 151 (70–3,154) | 0.4037 | |
| ALT (U/l) | 115 (20–733) | 75 (19–724) | 0.3673 | |
Data are shown as median (range). M, male; MC, male castrated; F, female; FS, female spayed; ND, not determined.
Clinical and laboratory findings
The clinical and laboratory findings are presented in Table 1. Dogs in both groups had chronic gastrointestinal signs before the referral; the median duration of clinical signs was 6 months (1–48 months) in clonality-positive group and 5 months (range, 1–24 months) in clonality-negative group. The median CIBDAI score was 8 points (range, 2–13 points) in clonality-positive group and 7 points (range, 5–13 points) in clonality-negative group. Using CIBDAI scores, both groups were classified as having moderate disease. The median CCECAI score in the clonality-positive group was 8 points (range, 2–17 points), which was categorized moderate disease. The median CCECAI score was 10 points (range, 5–14 points) in the clonality-negative group, which was categorized as severe disease. Six dogs in each group had hypoproteinemia (defined as total protein <5.0 g/dl), while nine dogs in the clonality-positive group and all dogs in the clonality-negative group had hypoalbuminemia (defined as albumin <2.6 g/dl). There were no statistically significant differences in clinical and laboratory parameters between groups.
Histopathology
On histopathological examination, intense intraepithelial and lamina propria infiltration of small lymphocytes was commonly observed, especially in the duodenum. Epithelial ulceration, villous blunting and fusion, and lacteal dilation were also noted. In all dogs, the duodenum was one of the most severely affected site. As the consequence, histopathological evaluation and PARR analysis were performed using samples obtained from the duodenum in every dog. Marked infiltration of small lymphocytes into the epithelia indicating epitheliotropism was observed in 12 dogs. Twenty of 22 dogs were judged as severe on histopathological examination. No significant differences were detected in the number of the dogs with severe lesions on histopathology between groups (P=0.1558; Table 2).
Table 2. Severity of histopathological lesions in the gastrointestinal tract, and presence or absence of epitheliotropism of lymphocytes, in clonality-positive and clonality-negative Shiba dogs.
| Clonality-positive group (n=13) | Clonality-negative group (n=9) | ||
|---|---|---|---|
| Severity of histopathological lesion | |||
| Normal−mild | 0 | 0 | |
| Moderate | 0 | 2 (22%) | |
| Severe | 13 (100%) | 7 (78%) | |
| Presence of epitheliotropism | |||
| Yes | 10 (77%) | 2 (17%) | |
| No | 3 (23%) | 7 (83%) | |
Data are shown as no. of dogs (%).
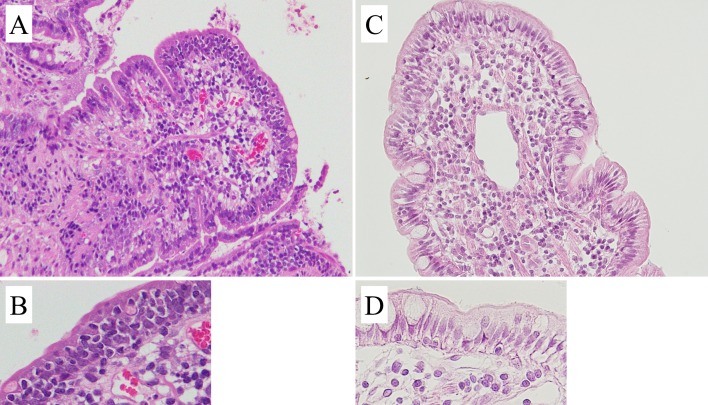
During analysis, we noted that clonality-positive dogs were more likely to also be epitheliotropism-positive, when compared to clonality-negative dogs. To investigate the relationship between PARR analysis and epitheliotropism, we classified cases as either epitheliotropism-positive (n=12) or epitheliotropism-negative (n=10), and performed additional analyses. Representative histopathological images are shown in Fig. 1. No significant differences were identified in gender, age, clinical signs or laboratory findings between the epitheliotropism-positive and -negative groups (data not shown). There was a significant association between the clonality-positive group and the epitheliotropism-positive group (P=0.027; Table 2).
Fig. 1.
Representative sections of duodenal mucosa obtained from two different Shiba dogs, classified as epitheliotropism-positive and epithelio-tropism-negative. Numerous small lymphocytes and some plasma cells are seen in lamina propria of both sections, supporting the diagnosis of severe lymphoplasmacytic enteritis. (A, B) Dense invasion of small lymphocytes into epithelium, suggesting epitheliotropic behavior. This dog was classified as epitheliotropism-positive. (C, D) Only a few small lymphocytes are seen in intraepithelial region, and this dog was classified as epitheliotropism-negative (Hematoxylin and eosin stain, × 200 and × 400).
Outcome
Prednisolone (0.5–2 mg/kg/day) was administered to all dogs as an initial therapy. Cyclosporine was used for two dogs in clonality-positive group and one dog in clonality-negative group. Chlorambucil was prescribed for four dogs in clonality-positive group and one dog in clonality-negative group. Concurrent immunosuppressive drugs were more frequently used in the clonality-positive group, although no significant differences were identified (P=0.3802). Some treatments including modified diet (either fat-restricted, low-residual, protein-hydrolyzed or hypoallergenic) or antibiotics (metronidazole or tylosin) and other drugs were prescribed according to the practitioner’s preference, and the variety of the treatments precluded statistical analysis.
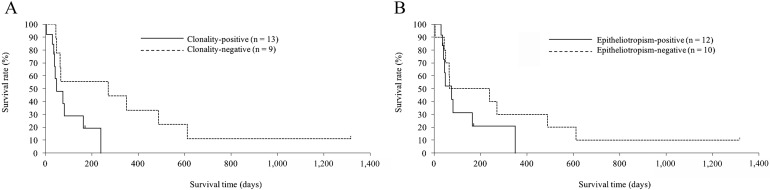
Eighteen of twenty-two dogs became refractory to treatment and died of disease progression. The remaining four dogs were lost to follow-up, after 37, 38, 171 and 1,316 days. No dogs were euthanized. The results of survival analysis using PARR analysis and presence of epitheliotropism are shown in Fig. 2. The median overall survival time for clonality-positive dogs was 48 days (range, 4–239 days) and for clonality -negative dogs was 271 days (range, 45–1,316 + days). The median overall survival time for epitheliotropism-positive dogs was 76 days (range, 30–349 days) and for epitheliotropism-negative dogs was 239 days (range, 4–1,316 + days). Statistical analysis revealed that clonality-positive results were associated with a significantly shorter survival time (Fig. 2A; P=0.036). In contrast, the presence or absence of epitheliotropism did not significantly affect survival time (Fig. 2B; P=0.223), although there was a tendency for epitheliotropism-positive group to have worse outcome.
Fig. 2.
Kaplan-Meier survival curves for Shiba dogs with chronic enteropathy. (A) Comparison between the clonality-positive and clonality-negative groups. Clonality was determined by PARR analysis. Survival time was significantly shorter in the clonality-positive group, when compared to the clonality-negative group (P=0.036). (B) Comparison between epitheliotropism-positive and -negative groups. Epitheliotropism was assessed by histopathology. Survival time was not statistically different between groups (P=0.223).
DISCUSSION
On PARR analysis, 59% Shiba dogs with CE had clonal rearrangement of antigen receptor gene, primarily a monoclonal rearrangement TCRγ gene. In addition, there was a significant correlation between clonal results, on PARR analysis, and epitheliotropism, on histopathology. In general, clonal population of lymphocytes, and epitheliotropism, are thought to be distinctive features of intestinal lymphoma, especially of the T-cell phenotype [7, 18]. Furthermore, dogs with clonal rearrangement had poorer prognosis than those without clonal rearrangement. We speculate that Shiba dogs showing both clonality and epitheliotropism could appropriately be diagnosed with intestinal small cell lymphoma of T-cell type.
In a previous report, clonality was detected in 51% of all dogs with CE, and 60% of dogs categorized as marked CE [13]. These detection rates were similar to our results, but the survival time reported in that study was not significantly different between dogs with and without clonal rearrangements. This contradiction might be explained by several factors. In the previous study, detection of clonality on PARR was not significantly associated with the presence of epitheliotropism on histopathology, although the positive rate of clonality tended to be higher in those with epitheliotropism than in those without epitheliotropism. Furthermore, clonality was observed even in 29% of dogs with only mild CE. In contrast, there was statistically significant association between PARR results and epitheliotropism, and no dog with normal to mild histopathological lesion was included in our study. Those differences might contribute to the discrepancy in the prognostic relevance of clonal rearrangement on PARR analysis. We could not elucidate it clearly, but some breed specific factors might exist in CE of Shiba dogs.
In cats, small T-cell lymphoma is the most common intestinal lymphoma, and the cells may be indistinguishable from inflammatory mature lymphocytes [4, 18, 19, 27]. Differentiation of small cell lymphoma from IBD is challenging. Transmural invasion by small lymphocytes, and severe epitheliotropism, such as intraepithelial lymphocytic nests and plaques, are specific for feline small cell lymphoma. Identification of transmural invasion requires a surgically obtained full-thickness biopsy. Furthermore, some degree of epitheliotropism can be seen not only in lymphoma, but also in IBD. The addition of immunohistochemistry and PARR analysis to conventional histopathology may be useful for differentiating lymphoma and IBD in cats [4, 27]. In our retrospective study, samples were obtained only by endoscopy, and immunohistochemistry could not be performed routinely. Those factors might obscure precise diagnosis.
We have previously reported that Shiba dogs with CE have a significantly shorter survival time, when compared to the survival of other breeds of dogs with CE [22]. We believe that Shiba dogs with CE have two different types of disease: IBD and small cell lymphoma. Inclusion of lymphoma of this type could explain the poor prognosis of Shiba dogs with CE. Interestingly, when cases with clonal rearrangement were excluded, Shiba dogs with CE still had a shorter median survival time of 271 days, when compared to previously reported median survival time of >1 year [2, 8, 13, 20, 22, 24]. One possible explanation is that as the disease progresses, IBD in Shiba dogs transforms to small cell lymphoma. This hypothesis is supported for several reasons. First, some lymphoma-suspected cases have a long history of gastrointestinal signs, as long as 4 years, which is not characteristic if lymphoma was present from the onset of the clinical signs. The lack of statistically significant differences in clinical and laboratory findings between clonality-positive and clonality -negative dogs might further reinforce the indistinguishable, or continuous, nature of IBD and small cell lymphoma in Shiba dogs. Reduced diversity of immunoglobulins and T-cell receptor gene rearrangement was detected in some cases of canine IBD, and the reduced diversity on PARR analysis has a significant correlation with the severity of the histopathological lesions and increased risk of death [26]. Detection rate of clonal rearrangement of the antigen receptor increases with the severity of histopathological lesion in dogs with CE [13]. The observation of clonal expansion of specific lymphocytes suggests the existence of occult lymphoma or a transition from a reactive to a neoplastic lesion. Further examination is warranted regarding its pathogenesis of CE in Shiba dogs.
In humans, primary intestinal T-cell lymphoma is rare, and enteropathy-associated T-cell lymphoma (EATL) is the main T-cell type intestinal lymphoma [9, 21, 28]. This type of lymphoma is thought to develop as a result of irritation from chronic gastrointestinal inflammatory diseases. According to the 2008 World Health Organization (WHO) Classification, there are two types of EATL. Type 1 EATL is the predominant type, comprising 80–90% of patients, is related to celiac disease and is characterized by infiltration of monoclonal medium-large sized neoplastic lymphoid cells with epitheliotropism. Type 2 EATL is relatively rare and is not associated with celiac disease, and the predominant neoplastic lymphoid cells are small to medium-sized, with marked intraepithelial infiltration. The clinical course of both types is aggressive, with a median survival time of 10 months. From the morphological characteristics of small lymphocytic infiltration and the possibility of transformation from inflammatory bowel disease to small cell lymphoma, feline small intestinal lymphoma is described as feline EATL type 2. In this disease, the prognosis is better, and the median survival time is reported to be 29 months [19]. There are few established reports of canine EATL type 2. Based on morphological evaluation, immunohistochemistry and PARR analysis, two of 8 dogs with gastrointestinal lymphoma were diagnosed as having EATL type 2, suggesting an infrequent occurrence of this disease in dogs [6]. A previous study mentioned canine intestinal lymphoma of the small cell type, with median survival time of approximately 1 year, which was greater survival when compared to our results [20]. We propose, based on histopathological characteristics, aggressive clinical course, and possible association with CE, that small T-cell lymphoma in Shiba dogs could be considered as canine EATL type 2.
This report has inherent limitations due to the retrospective nature of the study design. Only a small number of the cases were identified, which can effect statistical power to detect significance difference. Not all histopathological slides were examined by the same pathologist, so inter-pathologist variation could exist [30]. Ideally, unified diagnostic criteria, such as the World Small Animal Veterinary Association (WSAVA) intestinal histopathology guidelines, should be utilized [29]. Treatment protocols were not standardized, which could influence survival time. Cyclosporine and chlorambucil were used more frequently in clonality-positive dogs and could reflect the severity of the disease in this group. The use of immunosuppressive drugs could impact the prognosis.
In conclusion, we found that more than half of Shiba dogs with CE were clonality-positive on PARR analysis, and most of these dogs exhibited epitheliotropism on histopathology. These cases might appropriately be diagnosed as small T-cell intestinal lymphoma. In addition, there are common clinical and pathogenic features with human EATL type 2. The pathogenesis and grave prognosis of Shiba dogs with CE may be associated with this type of lymphoma, although further investigation is warranted.
REFERENCES
- 1.Allenspach K.2013. Diagnosis of small intestinal disorders in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 43: 1227–1240, v. doi: 10.1016/j.cvsm.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allenspach K., Wieland B., Gröne A., Gaschen F.2007. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 21: 700–708. doi: 10.1111/j.1939-1676.2007.tb03011.x [DOI] [PubMed] [Google Scholar]
- 3.Avery A.2009. Molecular diagnostics of hematologic malignancies. Top. Companion Anim. Med. 24: 144–150. doi: 10.1053/j.tcam.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 4.Briscoe K. A., Krockenberger M., Beatty J. A., Crowley A., Dennis M. M., Canfield P. J., Dhand N., Lingard A. E., Barrs V. R.2011. Histopathological and immunohistochemical evaluation of 53 cases of feline lymphoplasmacytic enteritis and low-grade alimentary lymphoma. J. Comp. Pathol. 145: 187–198. doi: 10.1016/j.jcpa.2010.12.011 [DOI] [PubMed] [Google Scholar]
- 5.Burnett R. C., Vernau W., Modiano J. F., Olver C. S., Moore P. F., Avery A. C.2003. Diagnosis of canine lymphoid neoplasia using clonal rearrangements of antigen receptor genes. Vet. Pathol. 40: 32–41. doi: 10.1354/vp.40-1-32 [DOI] [PubMed] [Google Scholar]
- 6.Carrasco V., Rodríguez-Bertos A., Rodríguez-Franco F., Wise A. G., Maes R., Mullaney T., Kiupel M.2015. Distinguishing intestinal lymphoma from inflammatory bowel disease in canine duodenal endoscopic biopsy samples. Vet. Pathol. 52: 668–675. doi: 10.1177/0300985814559398 [DOI] [PubMed] [Google Scholar]
- 7.Coyle K. A., Steinberg H.2004. Characterization of lymphocytes in canine gastrointestinal lymphoma. Vet. Pathol. 41: 141–146. doi: 10.1354/vp.41-2-141 [DOI] [PubMed] [Google Scholar]
- 8.Craven M., Simpson J. W., Ridyard A. E., Chandler M. L.2004. Canine inflammatory bowel disease: retrospective analysis of diagnosis and outcome in 80 cases (1995−2002). J. Small Anim. Pract. 45: 336–342. doi: 10.1111/j.1748-5827.2004.tb00245.x [DOI] [PubMed] [Google Scholar]
- 9.Ferreri A. J. M., Zinzani P. L., Govi S., Pileri S. A.2011. Enteropathy-associated T-cell lymphoma. Crit. Rev. Oncol. Hematol. 79: 84–90. doi: 10.1016/j.critrevonc.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 10.Fukushima K., Ohno K., Koshino-Goto Y., Uchida K., Nomura K., Takahashi M., Nakashima K., Fujino Y., Tsujimoto H.2009. Sensitivity for the detection of a clonally rearranged antigen receptor gene in endoscopically obtained biopsy specimens from canine alimentary lymphoma. J. Vet. Med. Sci. 71: 1673–1676. doi: 10.1292/jvms.001673 [DOI] [PubMed] [Google Scholar]
- 11.Gentilini F., Calzolari C., Turba M. E., Bettini G., Famigli-Bergamini P.2009. GeneScanning analysis of Ig/TCR gene rearrangements to detect clonality in canine lymphomas. Vet. Immunol. Immunopathol. 127: 47–56. doi: 10.1016/j.vetimm.2008.09.014 [DOI] [PubMed] [Google Scholar]
- 12.German A. J., Hall E. J., Day M. J.2003. Chronic intestinal inflammation and intestinal disease in dogs. J. Vet. Intern. Med. 17: 8–20. doi: 10.1111/j.1939-1676.2003.tb01318.x [DOI] [PubMed] [Google Scholar]
- 13.Hiyoshi S., Ohno K., Uchida K., Goto-Koshino Y., Nakashima K., Fukushima K., Kanemoto H., Maeda S., Tsujimoto H.2015. Association between lymphocyte antigen receptor gene rearrangements and histopathological evaluation in canine chronic enteropathy. Vet. Immunol. Immunopathol. 165: 138–144. doi: 10.1016/j.vetimm.2015.03.009 [DOI] [PubMed] [Google Scholar]
- 14.Jergens A. E., Schreiner C. A., Frank D. E., Niyo Y., Ahrens F. E., Eckersall P. D., Benson T. J., Evans R.2003. A scoring index for disease activity in canine inflammatory bowel disease. J. Vet. Intern. Med. 17: 291–297. doi: 10.1111/j.1939-1676.2003.tb02450.x [DOI] [PubMed] [Google Scholar]
- 15.Kaneko N., Yamamoto Y., Wada Y., Shimokawa Miyama T., Hiraoka H., Itamoto K., Mizuno T., Nakaichi M., Takahashi T., Watari T., Okuda M.2009. Application of polymerase chain reaction to analysis of antigen receptor rearrangements to support endoscopic diagnosis of canine alimentary lymphoma. J. Vet. Med. Sci. 71: 555–559. doi: 10.1292/jvms.71.555 [DOI] [PubMed] [Google Scholar]
- 16.Kathrani A., Werling D., Allenspach K.2011. Canine breeds at high risk of developing inflammatory bowel disease in the south-eastern UK. Vet. Rec. 169: 635. doi: 10.1136/vr.d5380 [DOI] [PubMed] [Google Scholar]
- 17.Keller S. M., Vernau W., Moore P. F.2016. Clonality testing in veterinary medicine: A review with diagnostic guidelines. Vet. Pathol. 53: 711–725. doi: 10.1177/0300985815626576 [DOI] [PubMed] [Google Scholar]
- 18.Kiupel M., Smedley R. C., Pfent C., Xie Y., Xue Y., Wise A. G., DeVaul J. M., Maes R. K.2011. Diagnostic algorithm to differentiate lymphoma from inflammation in feline small intestinal biopsy samples. Vet. Pathol. 48: 212–222. doi: 10.1177/0300985810389479 [DOI] [PubMed] [Google Scholar]
- 19.Moore P. F., Rodriguez-Bertos A., Kass P. H.2012. Feline gastrointestinal lymphoma: mucosal architecture, immunophenotype, and molecular clonality. Vet. Pathol. 49: 658–668. doi: 10.1177/0300985811404712 [DOI] [PubMed] [Google Scholar]
- 20.Nakashima K., Hiyoshi S., Ohno K., Uchida K., Goto-Koshino Y., Maeda S., Mizutani N., Takeuchi A., Tsujimoto H.2015. Prognostic factors in dogs with protein-losing enteropathy. Vet. J. 205: 28–32. doi: 10.1016/j.tvjl.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 21.Nijeboer P., Malamut G., Mulder C. J., Cerf-Bensussan N., Sibon D., Bouma G., Cellier C., Hermine O., Visser O.2015. Enteropathy-associated T-cell lymphoma: improving treatment strategies. Dig. Dis. 33: 231–235. doi: 10.1159/000369542 [DOI] [PubMed] [Google Scholar]
- 22.Ohmi A., Ohno K., Uchida K., Nakayama H., Koshino-Goto Y., Fukushima K., Takahashi M., Nakashima K., Fujino Y., Tsujimoto H.2011. A retrospective study in 21 Shiba dogs with chronic enteropathy. J. Vet. Med. Sci. 73: 1–5. doi: 10.1292/jvms.10-0154 [DOI] [PubMed] [Google Scholar]
- 23.Ohmura S., Leipig M., Schöpper I., Hergt F., Weber K., Rütgen B. C., Tsujimoto H., Hermanns W., Hirschberger J.2015. Detection of monoclonality in intestinal lymphoma with polymerase chain reaction for antigen receptor gene rearrangement analysis to differentiate from enteritis in dogs. Vet. Comp. Oncol. 15: 194–207. [DOI] [PubMed] [Google Scholar]
- 24.Ohno K., Konishi S., Kobayashi S., Nakashima K., Setoguchi A., Fujino Y., Nakayama H., Tsujimoto H.2006. Prognostic factors associated with survival in dogs with lymphocytic-plasmacytic enteritis. J. Vet. Med. Sci. 68: 929–933. doi: 10.1292/jvms.68.929 [DOI] [PubMed] [Google Scholar]
- 25.Okanishi H., Sano T., Yamaya Y., Kagawa Y., Watari T.2013. The characteristics of short- and long-term surviving Shiba dogs with chronic enteropathies and the risk factors for poor outcome. Acta Vet. Scand. 55: 32. doi: 10.1186/1751-0147-55-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olivero D., Turba M. E., Gentilini F.2011. Reduced diversity of immunoglobulin and T-cell receptor gene rearrangements in chronic inflammatory gastrointestinal diseases in dogs. Vet. Immunol. Immunopathol. 144: 337–345. doi: 10.1016/j.vetimm.2011.08.011 [DOI] [PubMed] [Google Scholar]
- 27.Sabattini S., Bottero E., Turba M. E., Vicchi F., Bo S., Bettini G.2016. Differentiating feline inflammatory bowel disease from alimentary lymphoma in duodenal endoscopic biopsies. J. Small Anim. Pract. 57: 396–401. doi: 10.1111/jsap.12494 [DOI] [PubMed] [Google Scholar]
- 28.Sieniawski M. K., Lennard A. L.2011. Enteropathy-associated T-cell lymphoma: epidemiology, clinical features, and current treatment strategies. Curr. Hematol. Malig. Rep. 6: 231–240. doi: 10.1007/s11899-011-0097-7 [DOI] [PubMed] [Google Scholar]
- 29.Washabau R. J., Day M. J., Willard M. D., Hall E. J., Jergens A. E., Mansell J., Minami T., Bilzer T. W., WSAVA International Gastrointestinal Standardization Group. 2010. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J. Vet. Intern. Med. 24: 10–26. doi: 10.1111/j.1939-1676.2009.0443.x [DOI] [PubMed] [Google Scholar]
- 30.Willard M. D., Jergens A. E., Duncan R. B., Leib M. S., McCracken M. D., Denovo R. C., Helman R. G., Slater M. R., Harbison J. L.2002. Interobserver variation among histopathologic from dogs and cats. J. Am. Vet. Med. Assoc. 220: 1177–1182. doi: 10.2460/javma.2002.220.1177 [DOI] [PubMed] [Google Scholar]