Table 1. Optimization of the Reaction a .
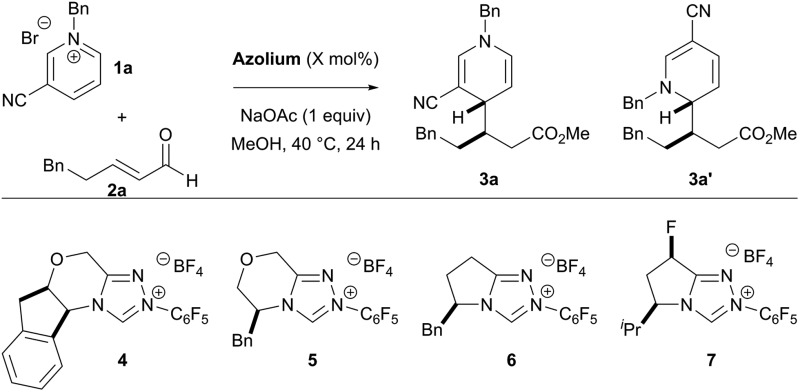
| ||||||
| Entry | Catalyst | X | Yield b [%] | 3a : 3a′ c | dr d | ee e [%] |
| 1 | 4 | 20 | 29 | 6 : 1 | 2 : 1 | 53 |
| 2 | 5 | 20 | 30 | 3 : 1 | 3 : 1 | 87 |
| 3 | 6 | 20 | 14 | 5 : 1 | 3 : 1 | 88 |
| 4 | 7 | 20 | 40 | 6 : 1 | 3 : 1 | 87 |
| 5 f | 7 | 20 | 87 | 6 : 1 | 3 : 1 | 87 |
| 6 f | 7 | 10 | 27 | 6 : 1 | 3 : 1 | 87 |
| 7 f , g | 7 | 10 | 61 | 6 : 1 | 3 : 1 | 87 |
aReaction conducted with 1.5 equiv. of 1a and 1.0 equiv. of 2a.
bCombined yield of 3a and 3a′; determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard.
cRegioisomeric ratio determined by 1H NMR of the unpurified reaction mixture.
dDiastereomeric ratio of 3a; determined by 1H NMR of the unpurified reaction mixture.
eEnantiomeric excess determined by HPLC using a chiral stationary phase.
fReaction set up in glovebox.
gReaction conducted in presence of 20 mol% acetic acid.
