Abstract
We mined novel uremic toxin (UT) metabolomics/gene databases, and analyzed the expression changes of UT receptors and UT synthases in chronic kidney disease (CKD) and cardiovascular disease (CVD). We made the following observations: 1) UTs represent only 1/80th of human serum small-molecule metabolome; 2) Some UTs are increased in CKD and CVD; 3) UTs either induce or suppress the expression of inflammatory molecules; 4) The expression of UT genes is significantly modulated in CKD patients, and coronary artery disease (CAD) patients; 5) The expression of UT genes is upregulated by caspase-1 and TNF-alpha pathways but is inhibited in regulatory T cells. These results demonstrate that UTs are selectively increased, and serve as danger signal-associated molecular patterns (DAMPs) and homeostasis-associated molecular patterns (HAMPs) that modulate inflammation. These results also show that some UT genes are upregulated in CKD and CAD via caspase-1/inflammatory cytokine pathways, rather than by purely passive accumulation.
Keywords: Uremia, Uremic Toxins, Danger Signal-Associated Molecular Patterns, Homeostasis-Associated Molecular Patterns, DAMPs, HAMPs, DAMP and HAMP receptors, Inflammation
2. INTRODUCTION
The incidence of chronic kidney disease (CKD) is increasing worldwide. Atherosclerosis-related cardiovascular disease (CVD) is a major cause of mortality in patients with CKD (1). We and others have previously shown that hyperlipidemia, along with other CVD stressors, such as hyperglycemia, hyperhomocysteinemia, and chronic kidney disease, promote atherosclerosis and vascular inflammation via several mechanisms (2–7). These mechanisms include endothelial cell (EC) activation and injury (2,8–10); mitochondrial reactive oxygen species (3); monocyte recruitment and differentiation (11,12); decreased regulatory T cells (13–15); impaired vascular repair ability of bone marrow–derived progenitor cells (16, 17); and downregulated histone modification enzymes (18).
CKD ranges from mild CKD to end-stage renal disease (ESRD), which requires therapies such as life-long hemodialysis or kidney transplantation (19). CKD is classified into 5 stages based on glomerular filtration rate (GFR, mL/min. per 1.7.3m2); ≥90mL/min (stage 1), 60–89mL/min (stage 2), 30–59mL/min (stage 3), 15–29mL/min (stage 4) and <15mL/min (stage 5). At stage 5, the patient develops ESRD, and requires dialysis. Tests for kidney function include creatinine clearance, creatinine levels, and blood urea nitrogen (BUN) assessment (MedlinePlus, NIH https://medlineplus.gov/kidneytests.html). CVD risk increases significantly according to the stages of CKD, ranging from 1.5.-fold in stage 2, to between 20 and 1,000-fold with ESRD (20). Indeed, CVD accounts for approximately 50% of deaths in patients receiving dialysis (21). These clinical data clearly demonstrate that CKD accelerates atherosclerosis, which along with its complications such as myocardial infarction, stroke and peripheral artery disease, are the leading cause of morbidity and mortality in the U.S., and account for 75% of all deaths from CVD (20, 22). The molecular and cellular mechanisms underlying CKD-accelerated atherosclerosis, especially the important issue of receptors in sensing uremic toxins (UTs), remain unknown (23).
It has been suggested that CKD uremic toxins (UTs), in combination with other risk factors, cause oxidative stress, low-grade inflammation with increased circulating cytokines and endothelial dysfunction (20, 23). One of the well-characterized UTs is carbamylated LDL (cLDL) (24). Urea spontaneously dissociates to form cyanate (OCN−), which modifies proteins in a process referred to as carbamylation. The active form of cyanate, isocyanic acid, reacts irreversibly with the amino acids in apolipoprotein B, the protein component of LDL to form cLDL (24). Protein carbamylation has been found in atherosclerotic plaque and serum level of cLDL is increased significantly in patients with ESRD. In addition, cLDL, but not native LDL, has been shown to have all of the major biological effects relevant to atherosclerosis, including EC injury and dysfunction by binding to oxLDL receptor (LOX-1), increased expression of cell adhesion molecules, monocyte adhesion, and vascular smooth muscle cell (VSMC) proliferation (24–26). However, the mechanistic link between sensing UTs and vascular inflammation remains unknown.
Cellular “receptors”, which can recognize the risk factors for vascular inflammation and atherogenesis, have been intensively researched. The role of pathogen-associated molecular patterns (PAMPs) and danger signal-associated molecular patterns (DAMPs) receptors has been characterized recently as bridging innate immune sensory systems for exogenous infectious agents and endogenous metabolic dangers to initiation of inflammation (27). More than 14 groups of endogenous metabolites have been proposed to act as danger signals via various DAMP recognition receptors to promote inflammation (28, 29). The Toll-like receptors (TLRs), mainly localized in the plasma membrane, recognize a variety of conserved microbial PAMPs and metabolic DAMPs, thereby functioning as PAMP and DAMP receptors, and promote inflammatory gene transcription. As we reported previously, for inflammation-privileged tissues, such as cardiovascular tissues in which inflammasome component genes are not constitutively expressed, TLRs work in synergy with upregulated cytosol-located sensing receptor families including NLRs (NOD (nucleotide binding and oligomerization domain)-like receptors) (30). In the cytosol, nucleus and extracellular compartment as we most recently reported, these inflammasome components and pro-caspase-1 assemble into a protein complex termed inflammasome, which subsequently activates caspase-1 after recognizing endogenous DAMPs (31). In this way, TLRs mediate upregulation, activation of a range of inflammatory genes and acceleration of vascular inflammation and atherosclerosis (2,32). After recognizing a paradox that classical DAMP receptors may not be able to bind with high affinity to all of the endogenous metabolite-derived danger signals, we proposed that endogenous metabolite-derived danger signals are conditional DAMPs, which together with our newly proposed homeostasis (anti-inflammatory)-associated molecular patterns (HAMPs), may use both intrinsic receptors and classical DAMP receptors to regulate inflammation (33). However, the issue of whether UTs serve as endogenous metabolite-derived danger signals to activate DAMPs receptors including TLRs and NLRs/inflammasome/caspase-1 remains unknown. To demonstrate a proof of principle that classical DAMP receptors play a critical role in accelerating CKD-promoted vascular inflammation, we recently reported that NLR-inflammasome caspase-1 pathway plays an essential role in sensing CKD-derived DAMPs, and in significantly promoting neointimal hyperplasia formation in carotid artery in 5/6 nephrectomy-induced CKD mouse model (6).
In this study, we collected 116 experimentally identified UTs and examined two novel hypotheses that: first, UTs can serve as conditional pro-inflammatory DAMPs, or anti-inflammatory HAMPs, and modulate inflammation; and second, in addition to passive accumulation due to decreased glomeruli filtration in CKD, elevation of UTs can be partially induced by classical DAMP receptors such as TLRs, NLR-inflammasome-activated caspase-1, and other pro-inflammatory cytokines as well as be inhibited by CD4+Foxp3+ regulatory T cells (Tregs). Using a novel database mining approach, our results have demonstrated for the first time that UTs are selectively increased, and serve as DAMPs and HAMPs to modulate inflammation (30,34); that UT genes including protein carried UT receptors and UT synthases can be upregulated in CKD and CAD presumably via caspase-1/inflammatory cytokine pathways; and that elevation of UTs does not result from purely passive accumulation. The findings have significantly improved our understanding of the molecular mechanisms underlying the roles of UTs in accelerating vascular inflammation and UT generation, which provide novel insights for the future development of novel therapeutics for CKD- and CKD-promoted cardiovascular disease and other diseases.
3. MATERIALS AND METHODS
3.1. Uremic toxins
We analyzed 116 experimentally verified UTs that were identified in recently published reports and review (35–37). The experimental method used in the identification of those UTs was mass spectrometry.
3.2. Expression profiles of uremic toxins and related enzymes and receptors in disease model
Gene expression profiles of the identified UTs were analyzed in 13 microarray datasets extracted from NIH-GEO database (http://www.ncbi.nlm.nih.gov/gds/) (Figure 1). The information regarding metabolite synthesis pathway enzymes was extracted from the Human Metabolome Database (http://www.hmdb.ca/). The information related to genes encoding protein/peptide-based UTs, enzymes, and receptors was obtained from the NCBI-Gene database (http://www.ncbi.nlm.nih.gov/gene/). The UTs which exist in the exosomes are examined in the ExoCarta database (http://www.exocarta.org/). The information of the UTs can be identified in NIH-NCBI-PubChem Database (https://pubchem.ncbi.nlm.nih.gov/). Specific samples were chosen as disease or treatment groups and parallel control. The number of samples was always greater than 3, except for the pooled samples. We selected the genes with significant expression changes (p<0.0.5) in the microarray dataset and examined the fold change of the genes of our interest. The genes with more than 1-fold expression change were defined as the upregulated genes while genes with their expression changes less than 1-fold were defined as downregulated genes.
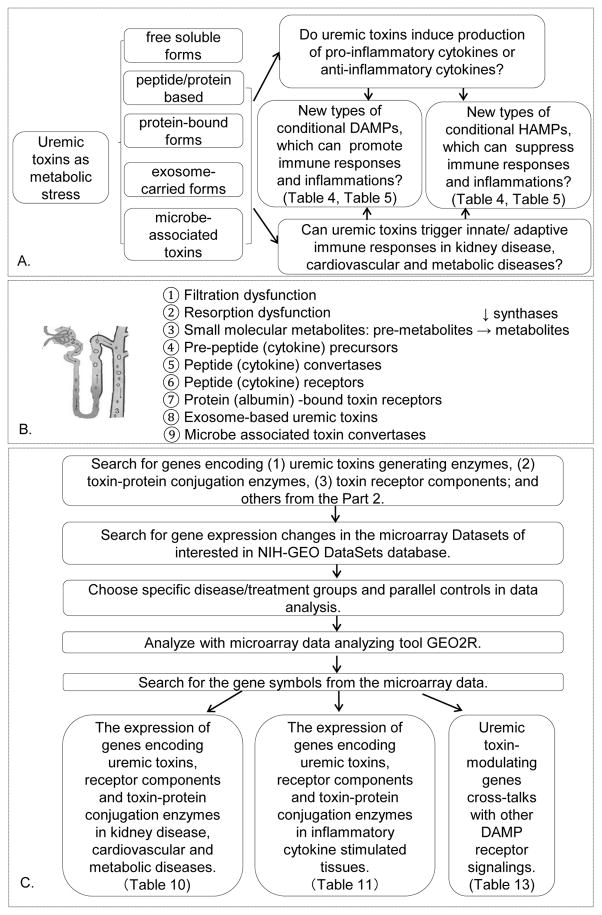
Figure 1.
Flow chart of database mining strategy and three parts of data organization. A. We propose a new paradigm that uremic toxins are conditional pro-inflammatory danger-associated molecular pattern molecules (DAMPs) or anti-inflammatory homeostasis-associated molecular pattern molecules (our newly proposed HAMPs). Uremic toxins identified were classified into five groups based on their molecular sizes, molecular structure, molecular carrier and sources. The supporting data for this new paradigm were presented in Tables 3, 4, 5 and Figure 2, respectively. B. Potential pathways for toxin synthase gene upregulation and its signaling components in pathophysiological conditions. The seven pathways from # 3 to #9 were examined in this study. C. Identified uremic toxin genes, their expression data and potential underlying mechanisms were analyzed through data-mining.
3.3. Ingenuity pathway analysis
In order to categorize clinical functions and molecular and cellular functions related to the identified genes in our microarray analysis, the Ingenuity Pathway Analysis (IPA, Ingenuity Systems, www.ingenuity.com) was used. The differentially expressed genes were identified and uploaded into IPA for analysis. The Core pathways analysis was used to identify molecular and cellular pathways.
4. RESULTS
4.1. Uremic toxins represent 1/80th of human serum small-molecule metabolome
To identify the molecular mechanisms of how CKD accelerates vascular inflammation, we hypothesized that CKD selectively accumulates a specific group of endogenous metabolites as UTs (Figure 2). We focused on analyzing the experimentally identified UTs. As shown in Table 1, 116 UTs have been identified (35–37), including four categories: 1) 53 small molecules (<500 Daltons); 2) 30 protein-bound molecules; 3) 39 middle-sized molecules, including protein/peptide-based (>500 Daltons); and 4) 15 microbe-generated toxins (38). Among 53 small-molecule toxins, only one receptor for inosine has been identified. Of note, the Human Metabolome Database identification numbers (IDs) for three small-molecule toxins were not found in the database; and the NIH-NCBI-PubChem Database IDs for five small-molecule toxins were not found in the database, suggesting these toxins are newly identified. In addition, 12 out of 30 protein-bound molecule toxins were found to have their own intrinsic receptors. Moreover, 20 out of 35 protein/peptide-based toxins had their own receptors, including several cytokines such as interleukin-18 (IL-18), IL-6, IL-1β, leptin, tumor necrosis factor-α (TNF-α). One of the microbe-generated UTs, pentosidine, can bind to the receptor for advanced glycation end products (RAGE) (39). As shown in Figure 3, those toxins, whose intrinsic receptors have not been identified, may also use classical DAMP receptors and nuclear receptors (for lipophilic toxins) to initiate inflammation-regulatory functions (29, 40–42). Furthermore, as shown in Table 2, our analysis on an exosome database (ExoCarta database; http://www.exocarta.org/) found that 6 out of 34 protein/peptide-based toxins have been found in exosomes in the plasma, suggesting that those toxins can promote/modulate the target cells for inflammation via exosome uptake mechanism with and without binding to their own receptors (43).
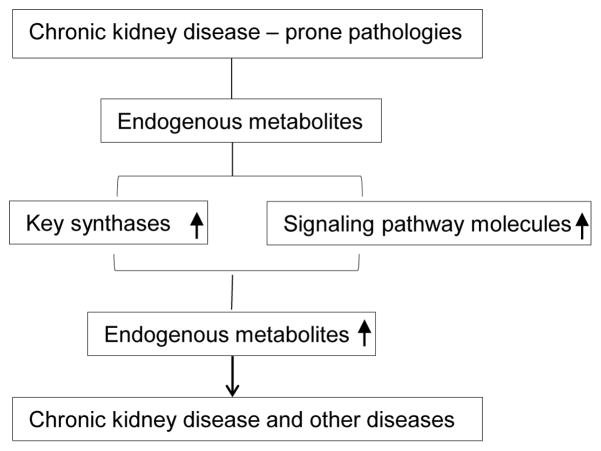
Figure 2.
Two novel hypotheses were examined on the expression levels of two sets of genes: First, uremic toxin generating enzymes (Table 6) and second, receptor complex components (Table 7). We examined these two hypotheses to address how chronic kidney disease prone pathologies increase endogenous metabolites.
Table 1.
116 uremic toxins, in four groups, experimentally identified in the plasma of patients with chronic kidney disease
| Toxin | Receptors | HMDB | PubChem ID | Gene ID |
|---|---|---|---|---|
| Group 1. small molecule (53) (<500Daltons) | ||||
| 1-Methyladenosine | -- | 03331 | 27476 | --1 |
| 1-Methylguanosine | -- | 01563 | 96373 | -- |
| 1-Methylinosine | -- | 02721 | 65095 | -- |
| 8-OH-2′Deoxyguanosine | -- | 03333 | -- | -- |
| Asymmetric dimethylarginine (ADMA) | -- | 01539 | 123831 | -- |
| Arabinitol | -- | 01851 | 439255 | -- |
| Argininic acid | -- | 03148 | 160437 | -- |
| Benzyl alcohol | -- | 03119 | 244 | -- |
| Creatine | -- | 00064 | 586 | -- |
| Creatinine | -- | 00562 | 588 | -- |
| Cytidine | -- | 00089 | 6175 | -- |
| Dimethylglycine | -- | 00092 | 673 | -- |
| Dimethylguanosine | -- | 04824 | 92919 | -- |
| Erythritol | -- | 02994 | 222285 | -- |
| Guanidine | -- | 01842 | 3520 | -- |
| Guanidinoacetate | -- | 00128 | 763 | -- |
| Guanidinosuccinate | -- | 03157 | 439918 | -- |
| Hypoxanthine | -- | 00157 | 790 | -- |
| Inosine | A2AR, A2R | 00195 | 6021 | -- |
| Malondialdehyde | -- | 06112 | 10964 | -- |
| Mannitol | -- | 00765 | 6251 | -- |
| Methylguanidine | -- | 01522 | 10111 | -- |
| Myoinositol | -- | 00211 | 892 | -- |
| N1-Methyl-2-pyridone-5-carboxamide | -- | 04193 | 69698 | -- |
| Nitrosodimethylamine | -- | 31419 | 6124 | -- |
| N2,N2-Dimethylguanosine | -- | 04824 | 92919 | -- |
| N4-Acetylcytidine | -- | 05923 | 107461 | -- |
| N6-carbamoyl-Threonyladenosine | -- | 41623 | -- | -- |
| N6-Methyladenosine | -- | 04044 | 102175 | -- |
| Orotic acid | -- | 00226 | 967 | -- |
| Orotidine | -- | 00788 | 92751 | -- |
| Oxalate | -- | 02329 | 971 | -- |
| Phenylacetylglutamine | -- | 06344 | 92258 | -- |
| Pseudouridine | -- | 00767 | 15047 | -- |
| Phenylethylamine | -- | 12275 | 1001 | -- |
| Sorbitol | -- | 00247 | 5780 | -- |
| Symmetric dimethylarginine (SDMA) | -- | 03334 | 169148 | -- |
| Thiocyanate | -- | 01453 | 9322 | -- |
| Taurocyamine | -- | 03584 | 68340 | -- |
| Threitol | -- | 04136 | 169019 | -- |
| Thymine | -- | 00262 | 1135 | -- |
| Trimethylamine | -- | 00906 | 1146 | -- |
| Uracil | -- | 00300 | 1174 | -- |
| Urea | -- | 00294 | 1176 | -- |
| Uric acid | -- | 00289 | 1175 | -- |
| Uridine | -- | 00296 | 6029 | -- |
| Xanthine | -- | 00292 | 1188 | -- |
| Xanthosine | -- | 00299 | 64959 | -- |
| α-N-Acetylarginine | -- | 04620 | -- | -- |
| γ-Guanidinobutyrate | -- | 03464 | 500 | -- |
| Nitrosomethylamine | -- | -- | 148811 | -- |
| α-keto-δ-Guanidinovaleriate | -- | -- | -- | -- |
| β-Guanidinopropionate | -- | -- | -- | -- |
| Group 2. protein-bound molecule (30) | ||||
| Carboxy methyl propyl furanpropionic acid (CMPF) | -- | 61112 | 123979 | -- |
| Urea | -- | 00294 | 1176 | -- |
| Homocysteine | -- | 00742 | 778 | -- |
| Hydroquinone | -- | 02434 | 785 | -- |
| Indole-3-acetate | -- | 00197 | 802 | -- |
| Indoxyl sulfate | -- | 00682 | 10258 | -- |
| Interleukin-18 | Il-18R | -- | -- | 3606 |
| Interleukin-6β | IL-6R | -- | -- | 3569 |
| Interleukin-1β | IL-1R | -- | -- | 3553 |
| Leptin | LEPR/OBR | -- | 90470904 | 3952 |
| Melatonin | MT1 MT2, RZR/ROR | 01389 | 896 | -- |
| Methylglyoxal | -- | 01167 | 880 | -- |
| p-Creso | -- | 01858 | 2879 | -- |
| Pentosidine | RAGE | 03933 | 119593 | -- |
| Phenol | -- | 00228 | 996 | -- |
| Phenylacetic acid | -- | 00209 | 999 | -- |
| Putrescine | -- | 01414 | 1045 | -- |
| Quinolinic acid | -- | 00232 | -- | -- |
| Retinol binding protein | -- | -- | -- | 5950 |
| Spermidine | -- | 01257 | 1102 | -- |
| Spermine | -- | 01256 | 1103 | -- |
| Tumor necrosis factor-α | TNFR | -- | -- | 7124 |
| 2-Methoxyresorcinol | -- | -- | 121805 | -- |
| 3-Deoxyglucosone | -- | -- | 114839 | -- |
| Fructoselysine | -- | -- | 49859675 | -- |
| Glyoxal | -- | -- | 7860 | -- |
| Kinurenine | AHR | -- | 846 | -- |
| Kinurenic acid | GPR35 | -- | 3845 | -- |
| Nε-Carboxymethyllysine | -- | -- | 123800 | -- |
| p-OHhippurate | -- | -- | -- | -- |
| Group 3. Middle molecule (4) (>500Daltons) | ||||
| Hyaluronic acid | GP85/CD44 | 02061 | 24728612 | -- |
| Octopamine | Octβ2R | 04825 | 4581 | -- |
| Dinucleoside polyphosphates | -- | -- | -- | -- |
| Uridineadenosine tetraphosphate (Up4Ab) | -- | -- | -- | -- |
| Protein/peptide based (35) (>500 Daltons) | ||||
| Substance P | GPCR, NK-1R, NK-2R, NK-3R | 01897 | 36511 | -- |
| Adrenomedullin | CRLR | -- | 56841671 | 133 |
| Calcitonin-gene related peptide (CGRP) | CALCRL, RAMP1 | -- | 56841902 | 796, 797 |
| Ghrelin | GHSR1a | -- | 44576256 | 51738 |
| Guanilin | -- | -- | 90488722 | 2980 |
| Leptin | LEPR/OBR | -- | 90470904 | 3952 |
| Orexin A | OX1R, OX2R | -- | 56842143 | 3060 |
| Parathyroid hormone | PTH1R | -- | 16129682 | 5741 |
| Uroguanylin | GC-C, GC-D | -- | 5488765 | 2981 |
| Vasoactive intestinal peptide | VPAC1, VPAC2 | -- | 16129679 | 7432 |
| Adiponectin | AdipoR1, AdipoR2 | -- | -- | 9370 |
| Atrial natriuretic peptide | NPR1, NPR2, NPR3 | -- | -- | 4878 |
| Basic fibroblast growth factor | bFGF-R1, bFGF-R2 | -- | -- | 2247 |
| Cholecystokinin | CCK1R, CCK2R | -- | -- | 885 |
| Clara cell protein | -- | -- | -- | 7356 |
| Complement factor D | -- | -- | -- | 1675 |
| Cystatin C | -- | -- | -- | 1471 |
| Endothelin | ETA, ETB1, ETB2, ETC | -- | -- | 1906 |
| Hepcidin | ferroportin | -- | -- | 744861 |
| Interleukin-18 | Il-18R | -- | -- | 3606 |
| Interleukin-6β | IL-6R | -- | -- | 3569 |
| Interleukin-1β | IL-1R | -- | -- | 3553 |
| Motiline | -- | -- | -- | 4295 |
| Neuropeptide Y | NPY1R, NPY2R, NPY3R, NPY4R, NPY5R, NPY6R, | -- | -- | 4852 |
| Retinol binding protein | -- | -- | -- | 5950 |
| Tumor necrosis factor-α | TNFR | -- | -- | 7124 |
| β2-Microglobulin | -- | -- | -- | 567 |
| κ-Ig Light chain | -- | -- | -- | 3514 |
| λ-Ig Light chain | -- | -- | -- | 3535 |
| Des-acylghrelin (DAG) | -- | -- | 90488789 | -- |
| Methionine-enkephalin | -- | -- | 6427062 | -- |
| δ-Sleep-inducing peptide | -- | -- | 3623358 | -- |
| degranulation-inhibiting protein-I, (DIP I) | -- | -- | -- | -- |
| β-Endorphin | -- | -- | -- | -- |
| β-Lipotropin | -- | -- | -- | -- |
| Group 4. microbe-associated toxins (15)2 (<500Daltons) | ||||
| Creatinine | -- | 00562 | 588 | -- |
| Guanidine | -- | 01842 | 3520 | -- |
| Urea | -- | 00294 | 1176 | -- |
| Indole-3-acetate | -- | 00197 | 802 | -- |
| Indoxyl sulfate | -- | 00682 | 10258 | -- |
| p-Creso | -- | 01858 | 2879 | -- |
| Phenol | -- | 00228 | 996 | -- |
| Phenylacetic acid | -- | 00209 | 999 | -- |
| Phenylacetylglutamine | -- | 06344 | 92258 | -- |
| Pentosidine3 | RAGE | 03933 | 119593 | -- |
| Putrescine3 | -- | 01414 | 1045 | -- |
| Spermidine3 | -- | 01257 | 1102 | -- |
| Spermine3 | -- | 01256 | 1103 | -- |
| Trimethylamine | -- | 00906 | 1146 | -- |
| Uric acid3 | -- | 00289 | 1175 | -- |
The IDs of the uremic toxins are not available in the databases.
they are normal metabolites of tryptophan, but the increased excretion in uremia is from bacterial degradation.
Reference PubMed ID: 19234110, PMID 19946322, PMID 25198138.
the five uremic toxins are generated both in body tissue and in bacterial
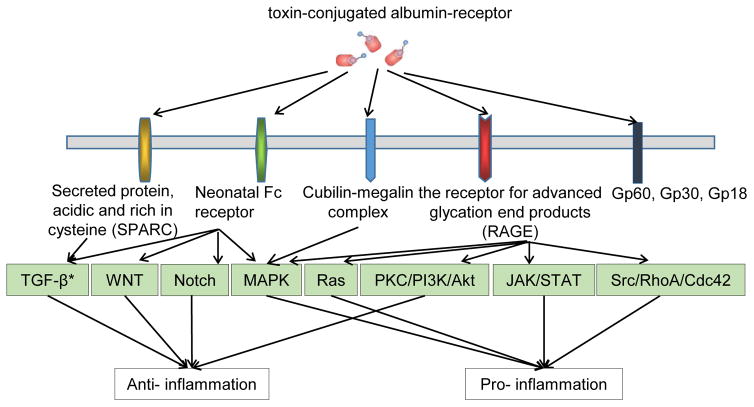
Figure 3.
Protein-bound uremic toxins could take use of five potential toxin-conjugated albumin-receptor pathways to either promote or suppress inflammation. There are two features for these five pathways: first, each receptor may signal several pathways; and second, downstream pathways connecting to each receptor can be shared. * TGF-β: Transforming growth factor beta; WNT: Wnt signaling pathways; Notch: Notch Signaling Pathway; MAPK: Mitogen-activated protein kinases; Ras: The Ras family; PKC/PI3K/Akt: Protein kinase C, phosphoinositide 3-kinases, protein kinase B; JAK/STAT: Janus kinase/signal transducer and activation of transcription; Src/RhoA/Cdc42: Proto-oncogene tyrosine-protein kinase Src, Ras homolog gene family, member A, Cell division control protein 42 homolog (Ref number 14517321, 25674083, 26055641, 25974754, 26925240).
Table 2.
6 uremic toxins have been identified in exosomes in plasma
| Uremic Toxin | Gene symbol | Gene ID |
|---|---|---|
| β2-Microglobulin | B2M | 567 |
| Complement factor D | CFD | 1675 |
| Cystatin C | CST3 | 1471 |
| κ-Ig Light chain | IGK | 50820 |
| λ-Ig Light chain | IGL | 3535 |
| Retinol binding protein | RBP4 | 5950 |
We first argued that if the generation of UTs results from accumulation of endogenous metabolites due to decreased glomeruli filtration in CKD, the compositions of UTs should be proportionally at least similar to, if not the same as, the human plasma metabolome. To our surprise, the most comprehensive Human Metabolome Database (HMDB) (version 3.6.) contains 41,993 metabolite entries, including both water-soluble and lipid-soluble metabolites as well as metabolites, that would be regarded as either abundant (>1μM) or relatively rare (<1nM) (http://www.hmdb.ca/). Obviously, not every metabolite appears in the human serum or plasma. Indeed, a recent report showed that 4,229 metabolites, roughly 10% of the total metabolites, have been identified in human serum metabolome (http://www.serummetabolome.ca)44. In addition, the Serum Metabolome database collected 4,651 small-molecule metabolites found in human serum (http://www.serummetabolome.ca/). Although these datasets did not result from the same studies, our analysis results, tentatively taken together, showed that roughly 1/80th, a very small fraction, of total human serum small-molecule metabolome were selectively accumulated in patients with CKD. If similar efficiencies were presumably achieved in identifying metabolites in human plasma and UTs with the current technologies, these analyses suggest that first, highly specific metabolites are highly selectively increased in the plasma of patients with CKD; and second, the high specificity of UTs accumulated in patients with CKD may not fully result from passive accumulation due to kidney malfunction in filtrating metabolites into urine. Instead, active mechanisms in synthesizing, processing, or converting these UTs may be the key regulating events for elevation of those toxins.
4.2. Classification of uremic toxins as DAMPs or HAMPs
We recently proposed a new paradigm that pathologically elevated endogenous metabolites can be categorized into either conditional pro-inflammatory danger-associated molecular patterns (DAMPs) or anti-inflammatory homeostasis-associated molecular patterns (HAMPs) based on the roles of these metabolites in regulating inflammation (33). To determine whether UTs are conditional DAMPs or HAMPs, we searched the Human Metabolome Database for the concentrations of toxins in physiological and pathological conditions. As shown in Table 3, among 69 UTs that can be found in the Human Metabolome Database, 24 (35%) UTs are unique to CKD, the remaining 45 (65%) UTs are shared with other diseases/CVD risk factors, including various cancers, smoking, hypertension, Alzheimer’s disease, cirrhosis, Canavan disease, etc. Among these 24 CKD UTs, 11 toxins had no reports on the physiological and pathological concentrations for comparison in the Human Metabolome Database. Seven CKD UTs were significantly increased more than 5-fold, including 3-Carboxy-4-methyl-5-propyl-2-furan propionate (CMPF, causing proximal tubular cell damage) (8-fold) (45); dimethylguanosine (altered RNA metabolism in patients with CKD) (14.2.-fold) (46); methylguanidine (26-fold); N2,N2-dimethylguanosine (14.2.-fold); p-Cresol (causing growth retardation) (>10-fold) (47); phenol (9.1.5-fold); and taurocyamine (inducing convulsive seizures; https://www.wikigenes.org/e/chem/e/68340.html) (7.8.-fold). The concentrations of two CKD toxins were actually decreased, including spermidine and spermine, which may cause the arrest in protein translation and cell growth (48). Of note, the concentration changes in other diseases may either be increased or decreased in comparison to those of physiological conditions. These results suggest that these UTs that are changed in other diseases with opposite directions may contribute differently to the pathogenesis of those diseases. Among 24 CKD-specific UTs, the pathological concentrations of 10 toxins are available for the analysis, which are all increased in the pathological conditions, suggesting that these UTs are the conditional DAMPs.
Table 3.
The supporting evidence 1 for classifying uremic toxins as conditional DAMPs or HAMPs: The uremic toxins are elevated in the plasma of patients with CKD and other diseases (69)
| Toxin | Physiological Concentration |
Pathological Concentration |
Pathological/Physiological concentration ratio |
Elevation in Other Disease | PMID |
|---|---|---|---|---|---|
| Metabolites elevated only in CKD (24) | |||||
| Benzyl alcohol | -- | -- | -- | -- | -- |
| CMPF | 4.6 ± 4.2 μM | 36.63 ± 20.81 μM | 7.96 | -- | -- |
| Dimethylglycine | 1.8–3.7 μM | 3.1–7.2 μM | 5.15 | -- | -- |
| Dimethyl guanosine | 0.031 ± 0.004 μM | 0.44 ± 0.09 μM | 14.19 | -- | -- |
| Guanidinoacetate | 2.8 ± 0.9 μM | 2.4 ± 0.7 μM | 0.86 | -- | -- |
| Hydroquinone | -- | -- | -- | -- | -- |
| Indoxyl sulfate | 14.0 ± 4.2 μM | 21.11 ± 12.20 μM | 1.51 | -- | -- |
| Melatonin | 0.000063 ± 0.000026 μM | -- | <15873 | -- | -- |
| Methylguanidine | 0.0–0.05 μM | 3.3 ± 1.3 μM | 132 | -- | -- |
| Nitrosodimethylamine | -- | -- | -- | -- | -- |
| N2,N2-Dimethylguanosine | 0.031 ± 0.004 μM | 0.44 ± 0.09 μM | 14.19 | -- | -- |
| N4-Acetylcytidine | -- | -- | -- | -- | -- |
| N6-Methyladenosine | -- | -- | -- | -- | -- |
| N6-carbamoyl-Threonyladenosine | -- | -- | -- | -- | -- |
| Orotidine | 149.0 ± 13.0 μM | -- | <0.00 | -- | -- |
| p-Cresol | -- | 9.9 ± 5.1 μM | >9.9 | -- | -- |
| Phenol | 6.38 ± 2.13 μM | 58.44 ± 39.32 μM | 9.16 | -- | -- |
| Phenylacetylglutamine | 3.34 ± 0.31 μM | -- | <0.30 | -- | -- |
| Phenylethylamine | -- | -- | -- | -- | -- |
| Spermidine | 10.3 ± 3.78 μM | 0.069 ± 0.053 μM | 0.00 | -- | -- |
| Spermine | 9.97 ± 3.26 μM | 0.0092 ± 0.0076 μM | 0.00 | -- | -- |
| Thiocyanate | 30.7 ± 28.8 μM | 32.02 ± 2.93 μM | 1.04 | -- | -- |
| Taurocyamine | 0.33 μM | 2.56 μM | 7.76 | -- | -- |
| Xanthosine | 5.08 ± 0.30 μM | -- | <0.20 | -- | -- |
| Metabolites also elevated in other diseases (45) | |||||
| 1-Methyladenosine | 0.10 ± 0.03 μM | 0.078 ± 0.031 μM | 0.07 | Cervical cancer | 7482520 |
| Cholangiocarcinoma | 7482520 | ||||
| Colorectal cancer | 7482520 | ||||
| Stomach cancer | 7482520 | ||||
| Hepatocellular carcinoma | 7482520 | ||||
| Leukemia | 7482520 | ||||
| Ovarian cancer | 7482520 | ||||
| 1-Methylguanosine | 0.046 ± 0.019 μM | 0.099 ± 0.021 μM | 2.15 | Perillyl alcohol administration for cancer treatment | 15607313 |
| 1-Methylinosine | 0.0680 ± 0.022 μM | -- | -- | Thyroid cancer | 9129323 |
| 8-OH-2′Deoxyguanosine | 0.002 ± 0.0008 μM | 0.0037 ± 0.00021 μM | 1.85 | Smoking | 18029489 |
| Asymmetric dimethylarginine (ADMA) | 0.28–0.42 μM | 4.35 ± 0.19 μM | 15.54 | Autosomal dominant polycystic kidney disease | 18215696 |
| Essential hypertension | 10218738 | ||||
| Arabinitol | 0.0–5.0 μM | 32.0–198.0 μM | 46 | Alzheimer’s disease | 8595727 |
| Ribose-5-phosphate isomerase deficiency | 14988808 | ||||
| Argininic acid | 0.015–0.44 μM | 0.015–0.5 μM | 1.14 | Cirrhosis | 7752905 |
| Creatine | 54.8 ± 21.0 μM | 33.8 ± 37.7 μM | 0.62 | Cirrhosis | 7752905 |
| Lung Cancer | 22157537 | ||||
| Rhabdomyolysis | 12089184 | ||||
| Creatinine | 82.6 ± 26.2 μM | 86.9 ± 44.5 μM | 1.05 | Canavan disease | 16139832 |
| Hyperoxalemia | 15353324 | ||||
| Paraquat poisoning | 9625050 | ||||
| Cytidine | 0.25±0.19 μM | 0.26 ± 0.13 μM | 1.04 | Canavan disease | 16139832 |
| Erythritol | 4.10 ± 1.64 μM | -- | <0.24 | Ribose-5-phosphate isomerase deficiency | 14988808 |
| Guanidine | 0.06–0.2 μM | 3.1 ± 1.1 μM | 23.85 | Cirrhosis | 7752905 |
| Guanidinosuccinate | 0.37–1.13 μM | 0.11 ± 0.106 μM | 0.30 | Cirrhosis | 7752905 |
| Hippuric acid | 16.74 ± 11.16 μM | 486.68 ± 344.36 μM | 29.07 | Lung cancer | 18953024 |
| Paraquat poisoning | 9625050 | ||||
| Homocysteine | 7.3–16.2 μM | 68.80 ± 15.53 μM | 5.86 | Alzheimer’s disease | 11959400 |
| Continuous ambulatory peritoneal dialysis | 11380380 | ||||
| Creutzfeldt-Jakob disease | 15711082 | ||||
| Dementia | 17384003 | ||||
| Hyaluronic acid | -- | 0.04–10.52 μM | >5.28 | Biliary atresia | 17875085 |
| Epilepsy | 12121313 | ||||
| Hepatitis | 17875085 | ||||
| Hypoxanthine | 11.02 ± 3.67 μM | 5.7 ± 0.4 μM | 0.52 | Canavan disease | 16139832 |
| Degenerative disc disease | 6656991 | ||||
| Hydrocephalus | 2611770 | ||||
| Lesch-Nyhan syndrome | 3148065 | ||||
| Lung Cancer | 18953024 | ||||
| Indole-3-acetate | 2.85 ± 1.71 μM | 13.70 ± 12.56 μM | 4.81 | Appendicitis | 11462886 |
| Irritable bowel syndrome | 9505884 | ||||
| Inosine | 0.20 ± 0.07 μM | 0.68 ± 0.47 μM | 3.4 | Canavan disease | 16139832 |
| Coronary artery disease | 10499868 | ||||
| Critical illnesses | 9663253 | ||||
| Degenerative disc disease | 6656991 | ||||
| Purine nucleoside phosphorylase deficiency | 8595732 | ||||
| Malondialdehyde | 0.69 ± 0.13 μM | 5.40 ± 0.30 μM | 7.83 | Parkinson’s disease | 17145675 |
| Smoking | 18029489 | ||||
| Mannitol | 34.0 ± 18.0 μM | 1.14–2.12 μM | 0.05 | AIDS | 8748311 |
| Alzheimer’s disease | 8595727 | ||||
| Cytochrome C oxidase deficiency | 7710082 | ||||
| Lung Cancer | 18953024 | ||||
| Ribose-5-phosphate isomerase deficiency | 14988808 | ||||
| Methylglyoxal | 0.44–0.74 μM | 2.4–3.6 μM | 5.08 | Diabetes mellitus type 2 | 18760976 |
| Myoinositol | 24.0 ± 7.8 μM | 23.0–24.0 μM | 0.98 | Alzheimer’s disease | 8595727 |
| Cachexia | 18953024 | ||||
| Ribose-5-phosphate isomerase deficiency | 1498808 | ||||
| N1-Methyl-2-pyridone-5-carboxamide | 9.00 ± 4.47 μM | 51.27 ± 23.66 μM | 5.70 | Pellagra | 17709435 |
| Octopamine | 0.0026 ± 0.0014 μM | 0.0026 ± 0.0024 μM | 1 | Cirrhosis | 3137238 |
| Hypertension | 8255371 | ||||
| Orotic acid | 0.89 ± 0.63 μM | 0.94 ± 0.78 μM | 1.06 | Canavan disease | 16139832 |
| Oxalate | 6.43 ± 1.06 μM | 47.2 ± 22.9 μM | 7.34 | Hemodialysis | 15353324 |
| Pentosidine | 0.14 ± 0.05 μM | 1.53 ± 0.79 μM | 10.93 | Alzheimer’s disease | 12498967 |
| Multi-infarct dementia | 12498967 | ||||
| Phenylacetic acid | 47.24 ± 5.866 μM | 3490.0 ± 330.0 μM | 73.88 | Phenylketonuria | 2091926 |
| Pseudouridine | 3.18 ± 0.99 μM | 16.70 ± 3.72 μM | 5.25 | Canavan disease | 16139832 |
| Putrescine | 0.214 ± 0.08 μM | 0.11 ± 0.09 μM | 0.51 | Pancreatic cancer | 2315288 |
| Quinolinic acid | 0.47 ± 0.047 μM | -- | <2.13 | AIDS | 9657528 |
| Anemia | 12964115 | ||||
| TraμMatic brain injury | 15206793 | ||||
| Sorbitol | 1.09 ± 0.37 μM | -- | <0.92 | Alzheimer’s disease | 8595727 |
| Substance P | 3.6e-6 ± 1.8e-6 μM | 4.9e-6 ± 2.7e-6 μM | 2.03 | Migraine | 17123735 |
| Symmetric dimethylarginine (SDMA) | 0.368–0.552μM | 2.08 ± 0.11 μM | 5.65 | Autosomal dominant polycystic kidney disease | 18215696 |
| Trimethylamine | 0.42 ± 0.12 μM | 1.38 ± 0.48 μM | 3.29 | Trimethylaminuria | 9246418 |
| Threitol | 0.0–5.0μM | 5.0–8.0μM | 3 | Ribose-5-phosphate isomerase deficiency | 14988808 |
| Thymine | -- | 1390.0 ± 150.0 μM | >1390.0 | Beta-ureidopropionase deficiency | 15385443 |
| Thymidine treatment | 6736109 | ||||
| Uracil | 2.10 ± 1.02 μM | 2.25 ± 0.98 μM | 1.07 | Canavan disease | 16139832 |
| Hypertension | 9816152 | ||||
| Urea | 6074.6 ± 2154.2 μM | 3500.0 ± 1500.0 μM | 0.58 | Cirrhosis | 7752905 |
| Meningitis | 15627241 | ||||
| Tuberculous meningitis | 15627241 | ||||
| Uric acid | 377.6 ± 82.6 μM | 400.0 ± 103.2 μM | 1.06 | Adenylosuccinate lyase deficiency | 15571235 |
| Bacterial meningitis | 17942520 | ||||
| Cachexia | 11320368 | ||||
| Canavan disease | 16139832 | ||||
| Degenerative disc disease | 6656991 | ||||
| Diabetes mellitus type 2 | 11887176 | ||||
| Impaired glucose tolerance | 11887176 | ||||
| Lesch-Nyhan syndrome | 15804753 | ||||
| Meningitis | 11805243 | ||||
| Multiple sclerosis | 11985629 | ||||
| Uridine | 2.90–3.30 μM | 7.7 ± 0.9 μM | 2.66 | Canavan disease | 16139832 |
| Degenerative disc disease | 6656991 | ||||
| Lesch-Nyhan syndrome | 3148065 | ||||
| Xanthine | 1.27 ± 0.78 μM | 2.2 ± 0.3 μM | 1.73 | Canavan disease | 16139832 |
| Degenerative disc disease | 6656991 | ||||
| Hydrocephalus | 2611770 | ||||
| Lesch-Nyhan syndrome | 3148065 | ||||
| α-N-Acetylarginine | 1.25 ± 0.28 μM | -- | <0.8 | Hyperargininemia | 3433275 |
| γ-Guanidinobutyrate | 0.013–0.055 μM | 0.013–0.09 μM | 1.51 | Cirrhosis | 7752905 |
| Mannitol | 34.0 ± 18.0 μM | 1.14–2.12 μM | 0.05 | AIDS | 8748311 |
| Alzheimer’s disease | 8595727 | ||||
| Cytochrome C oxidase deficiency | 7710082 | ||||
| Lung Cancer | 18953024 | ||||
| Ribose-5-phosphate isomerase deficiency | 14988808 | ||||
| Methylglyoxal | 0.44–0.74 μM | 2.4–3.6 μM | 5.08 | Diabetes mellitus type 2 | 18760976 |
| Myoinositol | 24.0 ± 7.8 μM | 23.0–24.0 μM | 0.98 | Alzheimer’s disease | 8595727 |
| Cachexia | 18953024 | ||||
| Ribose-5-phosphate isomerase deficiency | 1498808 | ||||
| N1-Methyl-2-pyridone-5-carboxamide | 9.00 ± 4.47 μM | 51.27 ± 23.66 μM | 5.70 | Pellagra | 17709435 |
| Octopamine | 0.0026 ± 0.0014 μM | 0.0026 ± 0.0024 μM | 1 | Cirrhosis | 3137238 |
| Hypertension | 8255371 | ||||
| Orotic acid | 0.89 ± 0.63 μM | 0.94 ± 0.78 μM | 1.06 | Canavan disease | 16139832 |
| Oxalate | 6.43 ± 1.06 μM | 47.2 ± 22.9 μM | 7.34 | Hemodialysis | 15353324 |
| Pentosidine | 0.14 ± 0.05 μM | 1.53 ± 0.79 μM | 10.93 | Alzheimer’s disease | 12498967 |
| Multi-infarct dementia | 12498967 | ||||
| Phenylacetic acid | 47.24 ± 5.866 μM | 3490.0 ± 330.0 μM | 73.88 | Phenylketonuria | 2091926 |
| Pseudouridine | 3.18 ± 0.99 μM | 16.70 ± 3.72 μM | 5.25 | Canavan disease | 16139832 |
| Putrescine | 0.214 ± 0.08 μM | 0.11 ± 0.09 μM | 0.51 | Pancreatic cancer | 2315288 |
| Quinolinic acid | 0.47 ± 0.047 μM | -- | <2.13 | AIDS | 9657528 |
| Anemia | 12964115 | ||||
| Traμmatic brain injury | 15206793 | ||||
| Sorbitol | 1.09 ± 0.37 μM | -- | <0.92 | Alzheimer’s disease | 8595727 |
| Substance P | 3.6e-6 ± 1.8e-6 μM | 4.9e-6 ± 2.7e-6 μM | 2.03 | Migraine | 17123735 |
| Symmetric dimethylarginine (SDMA) | 0.368–0.552μM | 2.08 ± 0.11 μM | 5.65 | Autosomal dominant polycystic kidney disease | 18215696 |
| Trimethylamine | 0.42 ± 0.12 μM | 1.38 ± 0.48 μM | 3.29 | Trimethylaminuria | 9246418 |
| Threitol | 0.0–5.0μM | 5.0–8.0μM | 3 | Ribose-5-phosphate isomerase deficiency | 14988808 |
| Thymine | -- | 1390.0 ± 150.0 μM | >1390.0 | Beta-ureidopropionase deficiency | 15385443 |
| Thymidine treatment | 6736109 | ||||
| Uracil | 2.10 ± 1.02 μM | 2.25 ± 0.98 μM | 1.07 | Canavan disease | 16139832 |
| Hypertension | 9816152 | ||||
| Urea | 6074.6 ± 2154.2 μM | 3500.0 ± 1500.0 μM | 0.58 | Cirrhosis | 7752905 |
| Meningitis | 15627241 | ||||
| Tuberculous meningitis | 15627241 | ||||
| Uric acid | 377.6 ± 82.6 μM | 400.0 ± 103.2 μM | 1.06 | Adenylosuccinate lyase deficiency | 15571235 |
| Bacterial meningitis | 17942520 | ||||
| Cachexia | 11320368 | ||||
| Canavan disease | 16139832 | ||||
| Degenerative disc disease | 6656991 | ||||
| Diabetes mellitus type 2 | 11887176 | ||||
| Impaired glucose tolerance | 11887176 | ||||
| Lesch-Nyhan syndrome | 15804753 | ||||
| Meningitis | 11805243 | ||||
| Multiple sclerosis | 11985629 | ||||
| Uridine | 2.90–3.30 μM | 7.7 ± 0.9 μM | 2.66 | Canavan disease | 16139832 |
| Degenerative disc disease | 6656991 | ||||
| Lesch-Nyhan syndrome | 3148065 | ||||
| Xanthine | 1.27 ± 0.78 μM | 2.2 ± 0.3 μM | 1.73 | Canavan disease | 16139832 |
| Degenerative disc disease | 6656991 | ||||
| Hydrocephalus | 2611770 | ||||
| Lesch-Nyhan syndrome | 3148065 | ||||
| α-N-Acetylarginine | 1.25 ± 0.28 μM | -- | <0.8 | Hyperargininemia | 3433275 |
| γ-Guanidinobutyrate | 0.013–0.055 μM | 0.013–0.09 μM | 1.51 | Cirrhosis | 7752905 |
Next, in order to verify UTs are conditional DAMPs or HAMPs, we examined our new hypothesis that UTs regulate inflammation, by either inducing or suppressing the expression of pro-inflammatory cytokines. To test this hypothesis, we searched for the experimental evidence that UTs can induce the expression of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-18, IL-6, monocyte chemoattractant protein-1 (MCP-1), adhesion molecules, nuclear factor-kB (NF-kB) signaling molecules or mitogen-activated protein kinases (MAPK) signaling molecules, etc. As shown in Table 4, among 92 free UTs, we found experimental reports showing that 32 UTs regulate inflammation in various cell types, with 20 promoting inflammation (as DAMPs, 62.5%) and 12 inhibiting inflammation (as HAMPs, 37.5.%). Moreover, as shown in Table 5, among 30 protein-bound UTs, we found via searching experimental reports that 19 protein-bound UTs regulate inflammation (63.3%) in various cell types, with 14 promoting inflammation as DAMPs (73.7%) and 5 inhibiting inflammation as HAMPs (26.3.%). These results suggest that regardless of whether UTs are bound to carrier proteins or not, UTs promote, more than inhibit, inflammation; and that more protein-bound UTs (73.7% versus 62.5%) than free UTs promote inflammation.
Table 4.
The supporting evidence 2 for classifying uremic toxins as DAMPs or HAMPs: Free uremic toxins either promote (DAMPs) or inhibit inflammation (HAMPs)
| Metabolite | Concentration | Cell type/tissue | Induced cytokines/signaling | Suppressed cytokines/signaling | PMID |
|---|---|---|---|---|---|
| Promoting inflammation (20) | |||||
| Asymmetric dimethylarginine (ADMA) | 3 μM 10 μM 30 μM |
Human monocytoid cells | NF–κB | -- | 18295546 |
| Basic fibroblast growth factor | 50μl of 50μg | Inbred male Lewis rats | ICAM-1, P-selectin, E-selectin | -- | 16507899 |
| Clara cell protein | 10 M | Human bronchiolar epithelium | -- | IL-2, IFN-γ | 7865218 |
| Cystatin C | -- | Hypertension patient blood | TNF-α, IL-6, CRP | -- | 20809110 |
| Endothelin | -- | -- | NF-κB, MAPKs, TNF-α, IL-1, | -- | 25288367 |
| Ghrelin | 1 ng/ml | HUVEC | -- | IL-1α, IL-1β, IL-6, TNF-α, IL-8, MCP-1 | 21565248 |
| Guanidinoacetate | 1.88 μM | Human blood | TNF-α | -- | 18048424 |
| Guanidinosuccinate | 8.27 μM | Human blood | -- | Neutrophil superoxide production, Natural killer cell response to interleukin-2 | 18048424 |
| Interleukin-18 | -- | -- | -- | 16470011 | |
| Interleukin-1β | -- | -- | L-1β | -- | 16470011 |
| Interleukin-6β | -- | -- | IL-6β | -- | 16470011 |
| Malondialdehyde | 50 μmol/L | Human peripheral blood lymphocytes (PBLCs) | IL-25, IL-6, IL-8, ICAM-1, PKC, p38MAPK, NF-κB, | -- | 22956781 |
| Methylguanidine | 1.91 μM | Human blood | TNF-α | -- | 18048424, 17324147 |
| Neuropeptide Y | 0.02 g/L | Y1-deficient mice | IL-12,TNF-α, NO, IL-4, adenylate cyclase-cAMP, NF-κB, COX-2, MAPK, PKA, phospholipase C, PKC, phosphatidyl inositol-3-kinase | IFN-γ | 23538492 |
| Parathyroid hormone | 46.3pg/mL | Human blood | CRP | -- | 24782595 |
| Retinol binding protein | -- | HRCEC and HUVEC | VCAM-1, ICAM-1, E-selectin, MCP-1, IL-6 | -- | 23071093 |
| Symmetric dimethylarginine (SDMA) | 1.5 μM, 3.0 μM, 6.0 μM, 12.0 μM, 36.0 μM | Human blood | Monocytic ROS production | -- | 19059932 |
| Substance P | 2.0 μM | Human mast cells | IL-8, TNF, VEGF | -- | 1701206 |
| Tumor necrosis factor-α | -- | -- | TNF-α | -- | 23095282 |
| Uric acid | -- | -- | VSMC, proliferation, MAPK, NF-κ B IL-1β | -- | 15660333, 21234729 |
| Inhibiting inflammation (12) | |||||
| 8-OH-2′Deoxyguanosine | 60 mg/kg | Bal b/c mice | -- | TNF-α, IL-6, IL-18, IL-12p70, NF-κB, c-Jun | 18037125 |
| Adiponectin | -- | Human aortic endothelial cells | IL-10 | TNF-α, VCAM-1, E-selectin, ICAM-1, IL-8, NF-κB, MEK/ERK signaling pathway, cAMP-PKA | 17343838 |
| Adrenomedullin | 20.0 ng/kg | Male CD mice | IL-10 | iNOS, NF-κB, TNF-α, IL-1β | 22685374 |
| Creatine | 0.5 mM, 5 mM | Human pulmonary endothelial cells | -- | ICAM-1 expression, E-selectin expression | 12812994 |
| Cholecystokinin | -- | kidney tissues of mice | -- | CD68, ICAM-1, TGF-β, TNF-α, NF-κB | 22357963 |
| Hyaluronic acid | 0.1mg/ml, 1mg/ml, 2mg/ml, 3mg/ml, 5mg/ml | Human rheumatoid arthritis synovial tissues | -- | IL-1-induced MMP-1 production, TNF-α, MAPK, NF-κ B, p38 | 20360891 |
| Inosine | 100mg/Kg | Cecal ligation and puncture mice | -- | TNF-α, IL-1β, IL-6, macrophage inflammatory protein-2 | 23355189 |
| Leptin | -- | ob/ob mice | IL-4 | TNF-α, IL-6, IL-1β, JAK-STAT, PI3K, ERK 1/2. | 16879738 |
| Orexin A | -- | -- | -- | IL-6 and TNF-α | 25884812 |
| Thiocyanate | 400.0 μM | Airway-targeted ENaC–overexpressing mice murine macrophage-like cells | -- | KC,IL-1b, TNF-α, | 25490247 |
| Uridine | 80 μl, 24 μg/ml | Male C57BL/6 mice bronchoalveolar lavage (BAL) fluid; human neutrophils | -- | IL-6, IL-8, TGF-β, ROS | 26369416 |
| Vasoactive intestinal peptide (VIP) | -- | Human lymph node immune cells | Foxp3, TGF-β | IL-6, TNF-α, IL-12, NO, TLR-2/TLR-4 expression, CXCL1 production | 23538492 |
Table 5.
Protein-bound uremic toxins promote or inhibit inflammation
| Metabolite | Concentration | Cell type/tissue | Induced cytokines/signaling | Suppressed cytokines/signaling | PMID |
|---|---|---|---|---|---|
| Promoting inflammation (14) | |||||
| Glyoxal | 500 μM | HUVEC | COX-2, ERK | 18343213 | |
| Homocysteine | 100 μM5-300μM | HEACR at VSMCs | NF-κB, Proliferation of VSMCs | -- | 17822365 |
| Hydroquinone | 10 μM, 100 μM, | Wistar rat | VCAM-1, ICAM-1, IL-1β, TNF-α,, NF-κB | -- | 21645265 |
| Indoxyl sulfate | 125 μg/mL, 250 μg/mL | HUVECR at VSMC | MAPK | Endothelial proliferation, wound repair | 14717914, 18941374 |
| Interleukin-18 | -- | -- | -- | 16470011 | |
| Interleukin-1β | -- | -- | L-1β | -- | 16470011 |
| Interleukin-6β | -- | -- | IL-6β | -- | 16470011 |
| Methylglyoxal | 56–420 μM | HUVEC | JNK, p38 MAPK | -- | 18842828 |
| p-Cresol | 10μg/mL, 25 μg/mL, 50 μg/mL | HUVEC | -- | endothelial proliferation, wound repair | 14717914 |
| Pentosidine | 229 pmol/ml | Human blood | NF-κB, IL-6 | -- | 15580352 |
| Phenol | 50 μM | Human Caco-2 cells | IL-6, IL-8, MCP-1 | -- | 20816778 |
| Phenylacetic acid | 5.0 mM | Rat VSMC | iNOS | -- | |
| Retinol binding protein | -- | HRCEC and HUVEC | VCAM-1, ICAM-1, E-selectin, MCP-1, IL-6 | -- | 23071093 |
| Tumor necrosis factor-α | -- | -- | TNF-α | -- | 23095282 |
| Inhibiting inflammation (5) | |||||
| Leptin | -- | ob/ob mice | IL-4 | TNF-α, IL-6, IL-1β, JAK-STAT, PI3K, ERK 1/2. | 16879738 |
| Melatonin | 1 mg/kg/day | Male-accelerated mice | IL-10 | TNF-α, IL-1β | 20817086 |
| putrescine | 12.5 mg/Kg | Wistar strain rats liver | amount of malondialdehyde | Lipid peroxide | 20040939 |
| Spermidine | 3.5 mg/Kg | Wistar strain rats liver | amount of malondialdehyde | Lipid peroxide | 20040939 |
| Spermine | 2.5 mg/Kg | Wistar strain rats liver | amount of malondialdehyde | Lipid peroxide | |
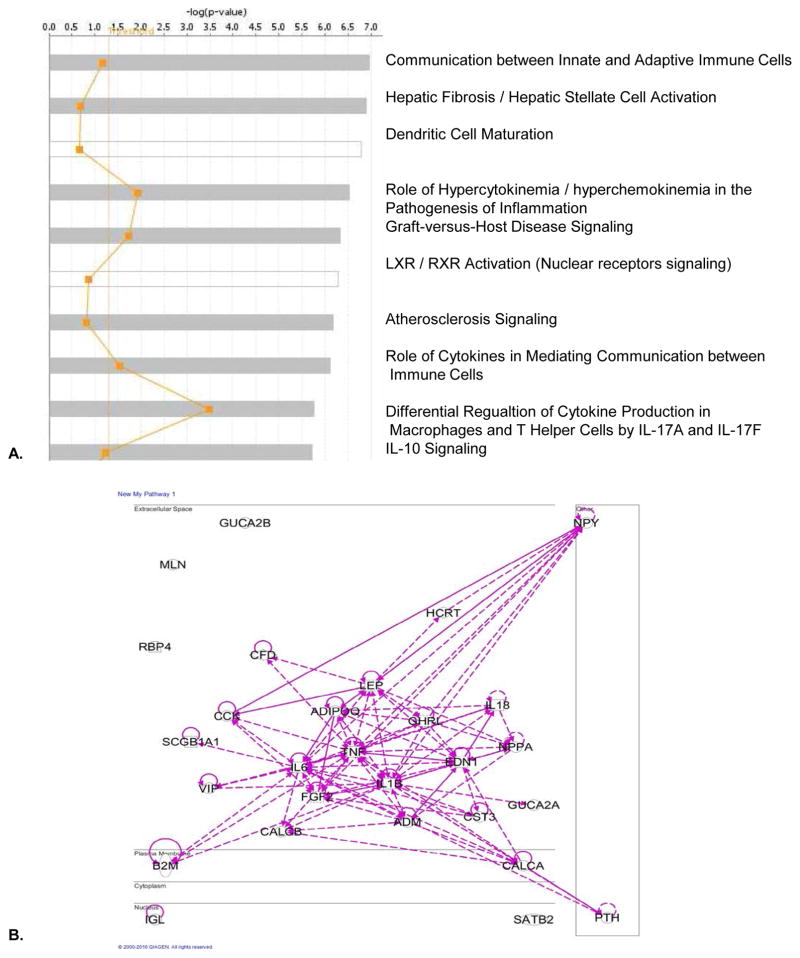
Finally, our Ingenuity Pathway Analysis (IPA) results of protein/peptide-based UTs indicated that the top ten pathways for those protein/peptide-based UTs (Figure 4) include: 1) communication between innate and adaptive immune cells; 2) hepatic fibrosis/hepatic stellate cell activation; 3) dendritic cell maturation; 4) role of hypercytokinemia/hyperchemokinemia in the pathogenesis of inflammation (influenza); 5) graft-versus-host disease signaling; 6) liver X nuclear receptor (LXR/RXR) activation (important regulators of cholesterol, fatty acid, and glucose homeostasis); 7) atherosclerosis signaling; 8) role of cytokines in mediating communications between immune cells; 9) differential regulation of cytokine production in macrophages and T helper cells by interleukin-17A (IL-17A) and IL-17F; and 10) IL-10 signaling. Once again, the IPA results strengthen our arguments that protein/peptide-based UTs have more pro-inflammatory than anti-inflammatory functions.
Figure 4.
The core analysis with the Ingenuity Pathway Analysis (IPA) suggest that peptide/protein-based uremic toxins play critical roles in promoting immune/inflammatory responses. A. On the left panel, top 10 pathways were identified for peptide/protein-based uremic toxins by The IPA. On the right panel, the relative significance scores were presented for the IPA selection of the top 10 pathways. B. The network shows the pathways of the peptide/protein based uremic toxins were interconnected.
4.3. Uremic toxins facilitate CAD in patients with CKD
Our above-described results indicate that roughly 1/80th, a very small fraction, of total human serum small-molecule metabolome was selectively accumulated in patients with CKD. The results suggest that the high specificity of UTs accumulated in patients with CKD may not result from passive accumulation due to kidney malfunction in filtrating metabolites into urine. We hypothesized that active mechanisms in synthesizing, processing, or converting these UTs may be the key regulating events for elevation of those toxins. To test this hypothesis, we first searched the toxin-generating enzymes. Among 69 UTs that can be found in the Human Metabolome Database, the 67 generating enzymes for 33 toxins can be found as shown in Table 6. In addition, for 30 protein-bound UTs that may mainly bind to serum albumin, albumin-bound UTs may initiate inflammation-regulatory signaling via binding to five potential receptor complexes and their signaling components, including glycoproteins (Gp60, Gp30 and Gp18) (49–51); secretedprotein acidic and rich in cysteine (SPARC, 8 genes) (50); neonatal Fc receptor (FcRn, 15 genes), cubilin-megalin (9 genes), and receptor for advanced glycation end products (RAGE, 23 genes), totaling 60 genes, as shown in Table 7 (50,52). Of note, some signaling components are shared among the receptor pathways. Moreover, as shown in Table 8, among 34 protein/peptide-based UTs, the convertases for generating 14 out of 34 UTs have been identified. Furthermore, since exosomes are identified as a potential key carrier for CKD-driven cardiovascular disease, we found that 28 out of 169 genes that have been identified in exosomes in the ExoCarta exosome database, which indicate these UTs can use exosome uptake mechanisms to initiate inflammation-modulating pathways as shown in Table 9 (53). Taken together, we collected 169 genes that generate UTs (Table 6) and mediate UT signaling (Tables 7, 8 and 9).
Table 6.
The uremic toxin generating enzymes may be the key regulators for elevation of the toxins in the plasma of patients with chronic kidney disease
| Toxin (33) | enzymes | Enzyme Gene Name | NCBI-Gene ID | PMID |
|---|---|---|---|---|
| Arabinitol | Aldose reductase | AKR1B1 | 231 | 25722213 |
| Aldo-keto reductase family 1 member B10 | AKR1B10 | 57016 | 25686905 | |
| Creatine | Guanidinoacetate N-methyltransferase | GAMT | 2593 | 26202197 |
| Cytidine | 5′-nucleotidase | NT5E | 4907 | 25677906 |
| Cytosolic 5′-nucleotidase 1B | NT5C1B | 93034 | 11690631 | |
| Cytosolic 5′-nucleotidase 1A | NT5C1A | 84618 | 19352542 | |
| 5′ (3′)-deoxyribonucleotidase, cytosolic type | NT5C | 30833 | 15136231 | |
| 5′ (3′)-deoxyribonucleotidase, mitochondrial | NT5M | 56953 | 24506201 | |
| Cytosolic purine 5′-nucleotidase | NT5C2 | 22978 | 25857773 | |
| Cytosolic 5′-nucleotidase 3 | NT5C3 | 101125212 | -- | |
| Dimethylglycine | Betaine--homocysteine S-methyltransferase 1 | BHMT | 635 | 25144858 |
| S-methylmethionine--homocysteine S-methyltransferase | BHMT2 | 23743 | 18457970 | |
| γ-Guanidinobutyrate | Glycine amidinotransferase, mitochondrial | GATM | 2628 | 24047826 |
| Guanidinoacetate | Glycine amidinotransferase, mitochondrial | GATM | 2628 | 24047826 |
| Hypoxanthine | Hypoxanthine-guanine phosphoribosyltransferase | HPRT1 | 3251 | 26050630 |
| Purine nucleoside phosphorylase | PNP | 4860 | 24107682 | |
| Inosine | 5′-nucleotidase | NT5E | 4907 | 25677906 |
| Cytosolic 5′-nucleotidase 1B | NT5C1B | 93034 | 11690631 | |
| Cytosolic 5′-nucleotidase 1A | NT5C1A | 84618 | 19352542 | |
| 5′ (3′)-deoxyribonucleotidase, cytosolic type | NT5C | 30833 | 15136231 | |
| 5′ (3′)-deoxyribonucleotidase, mitochondrial | NT5M | 56953 | 24506201 | |
| Adenosine deaminase | ADA | 56953 | 24506201 | |
| Cytosolic purine 5′-nucleotidase | NT5C2 | 22978 | 25857773 | |
| Adenosine deaminase CECR1 | CECR1 | 51816 | 25888558 | |
| Myoinositol | Alpha-galactosidase A | GLA | 2717 | 25468652 |
| Inositol monophosphatase 1 | IMPA1 | 3612 | 11959401 | |
| Inositol monophosphatase 2 | IMPA2 | 3613 | 21213002 | |
| Glycerophosphodiester phosphodiesterase 1 | GDE1 | 511573 | 21464471 | |
| Inositol monophosphatase 3 | IMPAD1 | 54928 | 22887726 | |
| N1-Methyl-2-pyridone-5-carboxamide | Aldehyde oxidase | AOX1 | 316 | 23857892 |
| Orotic acid | Dihydroorotate dehydrogenase (quinone), mitochondrial | DHODH | 1723 | 23216901 |
| Uridine 5′-monophosphate synthase | UMPS | 7372 | 22931617 | |
| Pseudouridine | Pseudouridine-5′-monophosphatase | HDHD1 | 617253 | 19393038 |
| Phenylethylamine | Aromatic-L-amino-acid decarboxylase | DDC | 1644 | 22597765 |
| Sorbitol | Alpha-galactosidase A | GLA | 2717 | 25468652 |
| Thiocyanate | 3-mercaptopyruvate sulfurtransferase | MPST | 4357 | 25336638 |
| Thiosulfate sulfurtransferase | TST | 7263 | 23399736 | |
| Thymine | Dihydropyrimidine dehydrogenase (NADP (+)) | DPYD | 1806 | 25410891 |
| Thymidine phosphorylase | TYMP | 1890 | 25304388 | |
| Uracil | Dihydropyrimidine dehydrogenase (NADP (+)) | DPYD | 1806 | 25410891 |
| Purine nucleoside phosphorylase | PNP | 4860 | 24107682 | |
| Thymidine phosphorylase | TYMP | 1890 | 25304388 | |
| Uridine phosphorylase 1 | UPP1 | 7378 | 208568792 | |
| Uridine phosphorylase 2 | UPP2 | 151531 | 1855639 | |
| Uracil phosphoribosyltransferase homolog | UPRT | 139596 | 17384901 | |
| Urea | Arginase-1 | ARG1 | 383 | 26030248 |
| Arginase-2, mitochondrial | ARG2 | 384 | 26054597 | |
| Agmatinase, mitochondrial | AGMAT | 79814 | 21803059 | |
| Probable allantoicase | ALLC | 55821 | 11054555 | |
| Uric acid | Xanthine dehydrogenase/oxidase | XDH | 7498 | 25463089 |
| Uridine | 5′-nucleotidase | NT5E | 4907 | 25677906 |
| Cytosolic 5′-nucleotidase 1B | NT5C1B | 93034 | 11690631 | |
| Cytosolic 5′-nucleotidase 1A | NT5C1A | 84618 | 19352542 | |
| Xanthine | Xanthine dehydrogenase/oxidase | XDH | 7498 | 25463089 |
| Hypoxanthine-guanine phosphoribosyltransferase | HPRT1 | 3251 | 26050630 | |
| Guanine deaminase | GDA | 9615 | 16953063 | |
| Purine nucleoside phosphorylase | PNP | 4860 | 24107682 | |
| Xanthosine | 5′-nucleotidase | NT5E | 4907 | 25677906 |
| Cytosolic 5′-nucleotidase 1B | NT5C1B | 93034 | 11690631 | |
| Cytosolic 5′-nucleotidase 1A | NT5C1A | 84618 | 19352542 | |
| 5′ (3′)-deoxyribonucleotidase, cytosolic type | NT5C | 30833 | 15136231 | |
| 5′ (3′)-deoxyribonucleotidase, mitochondrial | NT5M | 56953 | 24506201 | |
| Cytosolic purine 5′-nucleotidase | NT5C2 | 22978 | 25857773 | |
| Hippuric acid | Glycine N-acyltransferase | GLYAT | 10249 | 26149650 |
| Homocysteine | Putative adenosylhomocysteinase 3 | AHCYL2 | 23382 | 16865262 |
| Adenosylhomocysteinase | AHCY | 191 | 25248746 | |
| Putative adenosylhomocysteinase 2 | AHCYL1 | 10768 | 25237103 | |
| Hydroquinone | Serum paraoxonase/lactonase 3 | PON3 | 5446 | 22153698 |
| Serum paraoxonase/arylesterase 1 | PON1 | 5444 | 25966589 | |
| Serum paraoxonase/arylesterase 2 | PON2 | 5445 | 26056385 | |
| Indole-3-acetate | 4-trimethylaminobutyraldehyde dehydrogenase | ALDH9 | 223 | 11790142 |
| Alpha-aminoadipic semialdehyde dehydrogenase | A1ALDH7 | 501 | 26260980 | |
| Aldehyde dehydrogenase, mitochondrial | A1ALDH2 | 217 | 26153479 | |
| Fatty aldehyde dehydrogenase | ALDH3A2 | 224 | 25784589 | |
| Aldehyde dehydrogenase X, mitochondrial | ALDH1B1 | 219 | 21216231 | |
| Melatonin | Acetylserotonin O-methyltransferase | ASMT | 438 | 24881886 |
| Methylglyoxal | Aldose reductase | AKR1B1 | 231 | 25722213 |
| Putrescine | eroxisomal N (1)-acetyl-spermine/spermidine oxidase | PAOX | 196743 | 20405312 |
| Quinolinic acid | Nicotinate-nucleotide pyrophosphorylase (carboxylating) | QPRT | 23475 | 24038671 |
| Spermidine | Peroxisomal N (1)-acetyl-spermine/spermidine oxidase | PAOX | 196743 | 20405312 |
| Spermidine synthase | SRM | 6723 | 17585781 | |
| Spermine oxidase | SMOX | 54498 | 25174398 | |
| Spermine | Spermine synthase | SMS | 6611 | 23805436 |
| Spermidine synthase | SRM | 6723 | 17585781 | |
| Phenylacetic acid | Aldehyde dehydrogenase, dimeric NADP-preferring | ALDH3A1 | 218 | 24762960 |
| Aldehyde dehydrogenase family 1 member A3 | ALDH1A3 | 220 | 25684492 | |
| Aldehyde dehydrogenase family 3 member B2 | ALDH3B2 | 222 | 8890755 | |
| Aldehyde dehydrogenase family 3 member B1 | ALDH3B1 | 221 | 23721920 | |
| Trimethylamine | Flavin Containing Monooxygenase 1 | FMO1 | 2326 | 25634968 |
| Flavin Containing Monooxygenase 2 | FMO2 | 2327 | 25634968 | |
| Flavin Containing Monooxygenase 3 | FMO3 | 2328 | 25634968 | |
| Flavin Containing Monooxygenase 4 | FMO4 | 2329 | 25634968 | |
| Flavin Containing Monooxygenase 5 | FMO5 | 2330 | 25634968 |
See Figure 2 for the rationale.
Table 7.
The receptor complex components for protein-bound uremic toxins and their signal components may also be the key regulators for pathogenic signaling
| Protein | Gene | NCBI-Gene ID | PMID |
|---|---|---|---|
| Glycoprotein Gp60 | UL1 | 2657001 | 26925240 |
| Glycoprotein Gp30 | UL44 | 2952505 | 26925240 |
| Glycoprotein Gp18 | F857_gp18 | 14182318 | 26925240 |
| SPARC | P13K | 18708 | 14517321 |
| Akt | 207 | ||
| RhoA* | 387 | ||
| SMAD2 | 4087 | ||
| SMAD3 | 4088 | ||
| TAK1 | 7182 | ||
| TAB1 | 10454 | ||
| MKK4 | 841591 | ||
| FcRn | Numb | 8650 | 25674083 |
| α-adaptin | 101901253 | ||
| CDC42 | 998 | ||
| RhoA | 387 | ||
| PP2A*- | 843333 | ||
| Smart2/3 | -- | ||
| Ets* | 692446 | ||
| c-Jun* | 3725 | ||
| Fos* | 2353 | ||
| Elk* | 131096 | ||
| HIF1* | 3091 | ||
| STAT* | 6646 | ||
| CREB* | 1385 | ||
| Stathmin* | 3925 | ||
| PLA2* | 5320 | ||
| Cubilin-megalin complex | Ets | 692446 | 26055641 |
| c-Jun | 3725 | ||
| Fos | 2353 | ||
| Elk | 131096 | ||
| HIF1 | 3091 | ||
| STAT | 6646 | ||
| CREB | 1385 | ||
| Stathmin | 3925 | ||
| PLA2 | 5320 | ||
| RAGE | Bad | 572 | 22934052 |
| Caspase8 | 841 | ||
| Gsk3 | 2932 | ||
| MDM2 | 4193 | ||
| NF-κB | 4790 | ||
| PP2A | 843333 | ||
| Ets | 692446 | ||
| c-Jun | 3725 | ||
| Fos | 2353 | ||
| Elk | 131096 | ||
| HIF1 | 3091 | ||
| STAT | 6646 | ||
| CREB | 1385 | ||
| Stathmin | 3925 | ||
| PLA2 | 5320 | ||
| NFAT | 32321 | ||
| Sap1 | 2539285 | ||
| Max | 4149 | ||
| Myc | 4609 | ||
| p53 | 2768677 | ||
| CHOP | 1649 | ||
| MEF2 | 853342 | ||
| ATF-2 | 1386 |
Table 8.
14 out of 34 convertases in the generation of peptide/protein uremic toxins have been identified
| Peptide/Protein | Convertase | Convertase gene | Convertase gene ID | PMID |
|---|---|---|---|---|
| Adiponectin | Furin | FURIN | 5045 | 10433221 |
| PC7 | PCSK7 | 9159 | ||
| Atrial natriuretic peptide | PC1/3 | PCSK1 | 5122 | 17050541 |
| Calcitonin-gene related peptide (CGRP) | ADAM17 | ADAM17 | 6868 | 11733179 |
| Clara cell protein | PC1 | PCSK1 | 5122 | 14608596 |
| PC2 | PCSK2 | 5125 | ||
| PC5 | PCSK5 | 5126 | ||
| Des-acyl ghrelin (DAG) | corin | CORIN | 10699 | 15637153 |
| Endothelin | ECE | ECE1 | 1899 | 11067800 |
| Hepcidin | ICE | CASP1 | 834 | 8044845 |
| Interleukin-18 | caspase-1 | CASP1 | 834 | 12706898 |
| Interleukin-1β | caspase-1 | CASP1 | 834 | 12706898 |
| Neuropeptide Y | PC2 | PCSK2 | 5125 | 7750497 |
| Orexin A | Furin | FURIN | 5045 | 17905609 |
| Parathyroid hormone | ICE | CASP1 | 834 | 10449160 |
| Substance P | PC1/3 | PCSK1 | 5122 | 9405066 |
| PC2 | PCSK2 | 5125 | ||
| Tumor necrosis factor-α | TACE/ADAM17/CD156q | ADAM17 | 6868 | 11733179 |
| Adrenomedullin | -- | -- | -- | -- |
| Basic fibroblast growth factor | -- | -- | -- | -- |
| Cholecystokinin | -- | -- | -- | -- |
| Complement factor D | -- | -- | -- | -- |
| Cystatin C | -- | -- | -- | -- |
| Degranulation-inhibiting protein-I, (DIP I) | -- | -- | -- | -- |
| Ghrelin | -- | -- | -- | -- |
| Interleukin-6β | -- | -- | -- | -- |
| Leptin | -- | -- | -- | -- |
| Methionine-enkephalin | -- | -- | -- | -- |
| Motiline | -- | -- | -- | -- |
| Retinol binding protein | -- | -- | -- | -- |
| Uroguanylin | -- | -- | -- | -- |
| Vasoactive intestinal peptide | -- | -- | -- | -- |
| β2-Microglobulin | -- | -- | -- | -- |
| β-Endorphin | -- | -- | -- | -- |
| β-Lipotropin | -- | -- | -- | -- |
| κ-Ig Light chain | -- | -- | -- | -- |
| λ-Ig Light chain | -- | -- | -- | -- |
| δ-Sleep-inducing peptide | -- | -- | -- | -- |
Table 9.
28 of the 169 genes have been identified in exosomes, which regulate the uremic toxins, the convertase of uremic toxins, the generation enzyme genes and the receptor complex components for protein-bound uremic toxins and their signal components
| Chemicals | Gene symbol | Gene ID |
|---|---|---|
| Uremic toxins (6) | ||
| β2-Microglobulin | B2M | 567 |
| Complement factor D | CFD | 1675 |
| Cystatin C | CST3 | 1471 |
| κ-Ig Light chain | IGK | 50820 |
| λ-Ig Light chain | IGL | 3535 |
| Retinol binding protein | RBP4 | 5950 |
| Convertases of uremic toxins (2) | ||
| Furin | FURIN | 5045 |
| ECE | ECE1 | 1899 |
| Enzymes of uremic toxins (18) | ||
| Adenosyl homocysteinase | AHCY | 191 |
| Putative adenosylhomocysteinase 2 | AHCYL1 | 10768 |
| Aldose reductase | AKR1B1 | 231 |
| Aldo-keto reductase family 1 member B10 | AKR1B10 | 57016 |
| Aldehyde dehydrogenase family 3 member B1 | ALDH3B1 | 221 |
| 4-trimethylaminobutyraldehyde dehydrogenase | ALDH9A1 | 223 |
| Aldehyde oxidase | AOX1 | 316 |
| S-methyl methionine--homocysteine S-methyltransferase | BHMT2 | 23743 |
| Aromatic-L-amino-acid decarboxylase | DDC | 1644 |
| Hypoxanthine-guanine phosphoribosyltransferase | HPRT1 | 3251 |
| 3-mercaptopyruvate sulfurtransferase | MPST | 4357 |
| 5′ (3′)-deoxyribonucleotidase, cytosolic type | NT5C | 30833 |
| 5′-nucleotidase | NT5E | 4907 |
| Purine nucleoside phosphorylase | PNP | 4860 |
| Serum paraoxonase/arylesterase 1 | PON1 | 5444 |
| Serum paraoxonase/lactonase 3 | PON3 | 5446 |
| Nicotinate-nucleotide pyrophosphorylase (carboxylating) | QPRT | 23475 |
| Xanthine dehydrogenase/oxidase | XDH | 7498 |
| Receptor complex (2) | ||
| FcRn | CASP8 | 841 |
| RAGE | CDC42 | 998 |
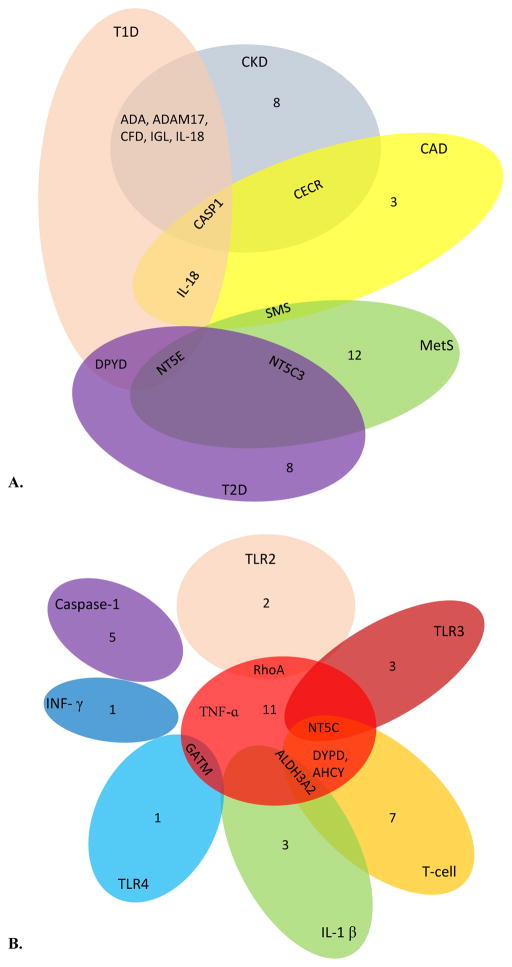
To examine our hypothesis that the expression of these 169 genes is partially modulated in various diseases (Figure 5) including CKD, we mined the microarray database in the NIH-GEO Datasets as shown in Table 10. Our analysis of microarray experimental data indicated that 14 UT genes were upregulated; and another 14 UT genes were downregulated in the CKD kidney tubules. In addition, we found that 7 UT genes were upregulated; and another 9 UT genes were downregulated in the adipose tissues of patients with coronary artery disease (CAD). The striking similarities have been noted in upregulated pro-inflammatory pathways, including pro-inflammatory caspase-1 and caspase-1 substrate IL-18 in both CKD kidney tubules and CAD adipose tissue, suggesting that the same upregulated pro-inflammatory pathways underlie the pathogenesis of CKD and CAD. Moreover, we observed that 3 UT genes were upregulated and another 16 UT genes were slightly downregulated in the peripheral blood cells of patients with metabolic syndrome. Furthermore, we identified that 4 UT genes were upregulated and another 12 UT genes were slightly downregulated in the liver of patients with type 2 diabetes, which were very similar to those observed in metabolic syndrome. Finally, we found that 9 UT genes were upregulated; and another 13 UT genes were slightly downregulated in the pancreas of patients with type 1 diabetes. Taken together, our results suggest that first, the upregulation of UT-generating enzymes, protein-bound UT receptors and their signaling components, and convertases for protein/peptide-based UTs in CKD-related diseases at least partially contribute to increased concentrations of UTs; and second, some inflammation-modulating genes in UT generation and signaling pathways are upregulated in CKD, CAD and other metabolic diseases, pointing out the potential cross-talking mechanisms underlying the roles of UTs in facilitating CAD in patients with CKD.
Figure 5.
The Venn diagram analyses demonstrate that various diseases may modulate uremic toxin generation in specific or shared manners; and that immune pathways regulate uremic toxin generations in specific or shared manners. A. Not only chronic kidney disease, but also other metabolic diseases upregulate uremic toxins. Chronic kidney disease shares the upregulation of uremic toxins with other metabolic diseases differentially. B. Innate immune sensor cytokines, and adaptive immune cell pathways differentially regulate the generations of uremic toxins.
Table 10.
The expressions of the genes encoding uremic toxins, receptor components and toxin-protein conjugation enzymes are more significantly upregulated in metabolic diseases than metabolite-targeted diseases
| Disease | Tissue or cell type | Gene | Fold change | Toxin | GEO Dataset ID | PMID |
|---|---|---|---|---|---|---|
| Control VS CKD | Human kidney tubules | Upregulated gene | GSE48944 | 24098934 | ||
| ALDH1B | 0.72 | Indole-3-acetate | ||||
| CALCA | 0.82 | CGRP | ||||
| CCK | 0.86 | Cholecystokinin | ||||
| DDC | 0.75 | Phenylethylamine | ||||
| DHODH | 0.72 | Orotic acid | ||||
| FMO4 | 0.57 | Trimethylamine | ||||
| GLYAT | 0.47 | Hippuric acid | ||||
| HAMP | 0.80 | Hepcidin | ||||
| MDM2 | 0.86 | Signal components | ||||
| NPPA | 0.75 | Atrial natriuretic peptide | ||||
| NT5M | 0.78 | Xanthosine/Cytidine | ||||
| PCSK2 | 0.77 | Substance P | ||||
| PON1 | 0.64 | Hydroquinone | ||||
| PON3 | 0.62 | Hydroquinone | ||||
| RBP4 | 0.36 | Retinol binding protein | ||||
| Downregulated gene | ||||||
| ADA | 1.42 | Inosine | ||||
| ADAM17 | 1.29 | CGRP | ||||
| ALDH1A | 1.98 | Phenylacetic acid | ||||
| B2M | 2.31 | β2-Microglobulin | ||||
| CASP1 | 2.31 | Interleukin-1β/IL-18 | ||||
| CECR1 | 1.85 | Inosine | ||||
| CFD | 1.72 | Complement factor D | ||||
| FMO3 | 1.52 | Trimethylamine | ||||
| HDHD1 | 1.53 | Pseudouridine | ||||
| IGK | 4.56 | κ-Ig Light chain | ||||
| IGL | 5.38 | λ-Ig Light chain | ||||
| IL18 | 1.65 | Interleukin-18 | ||||
| PCSK5 | 1.14 | Clara cell protein | ||||
| PON2 | 1.60 | Hydroquinone | ||||
| RHOA | 1.84 | Signal components | ||||
| Control VS Coronary Artery Disease | Human Epicardial Adipose Tissue and Subcutaneous Adipose Tissue) | Upregulated gene | GSE64566 | -- | ||
| ALDH7A1 | 0.86 | Indole-3-acetate | ||||
| ALDH9A1 | 0.77 | Indole-3-acetate | ||||
| CDC42 | 0.91 | Signal components | ||||
| GAMT | 0.92 | Creatine | ||||
| HCRT | 0.93 | Orexin | ||||
| PAOX | 0.90 | Putrescine/Spermidine | ||||
| PON2 | 0.88 | Hydroquinone | ||||
| PON3 | 0.89 | Hydroquinone | ||||
| TST | 0.85 | Thiocyanate | ||||
| Downregulated gene | ||||||
| ADIPOQ | 1.31 | Adiponectin | ||||
| AHCYL1 | 1.10 | Homocysteine | ||||
| CASP1 | 1.15 | Interleukin-1β | ||||
| CECR | 1.30 | Inosine | ||||
| 1IL18 | 1.11 | Interleukin-18 | ||||
| PCSK7 | 1.12 | Adiponectin | ||||
| SMS | 1.10 | Spermine | ||||
| Control VS Metabolic Syndrome | Human peripheral blood | Upregulated gene | GSE23561 | 21368773 | ||
| HAMP | 1.10 | Hepcidin | ||||
| NUMB | 1.04 | Signal components | ||||
| PCSK7 | 1.14 | Adiponectin | ||||
| Downregulated gene | ||||||
| AKR1B1 | 0.97 | Arabinitol | ||||
| AKR1B10 | 0.98 | Arabinitol | ||||
| ALDH1B1 | 0.98 | Indole-3-acetate | ||||
| ALDH3B1 | 0.97 | Phenylacetic acid | ||||
| ALDH9A1 | 0.98 | Indole-3-acetate | ||||
| ALLC | 0.98 | Urea | ||||
| CST3 | 0.97 | Cystatin C | ||||
| GHRL | 0.87 | Ghrelin | ||||
| HPRT1 | 0.98 | Xanthine/Hypoxanthine | ||||
| NT5C1A | 0.98 | Uridine/Xanthosine/Cytidine | ||||
| NT5C2 | 0.98 | Cytidine/Inosine/Xanthosine | ||||
| NT5C3 | 0.98 | Cytidine | ||||
| NT5E | 0.97 | Xanthosine/Cytidine | ||||
| SMOX | 0.96 | Spermidine | ||||
| SMS | 0.97 | Spermine | ||||
| UMPS | 0.98 | Orotic acid | ||||
| Control VS Type 2 Diabetes | Human liver | Upregulated gene | GSE23343 | 21035759 | ||
| CALCB | 2.14 | Calcitonin-gene related peptide (CGRP) | ||||
| HCRT | 3.72 | Orexin | ||||
| NT5C2 | 1.42 | Cytidine/Inosine/Xanthosine | ||||
| Downregulated gene | ||||||
| ADA | 0.63 | Inosine | ||||
| ADAM17 | 0.71 | CGRP | ||||
| ALDH1A3 | 0.44 | Phenylacetic acid | ||||
| CASP1 | 0.66 | Interleukin-1β/IL-18 | ||||
| CDC42 | 0.68 | Signal components | ||||
| CFD | 0.60 | Complement factor D | ||||
| DPYD | 0.68 | Thymine/Uracil | ||||
| FMO2 | 0.50 | Trimethylamine | ||||
| IGL | 0.47 | λ-Ig Light chain | ||||
| IL18 | 0.33 | Interleukin-18 | ||||
| MAX | 0.45 | Signal components | ||||
| MDM2 | 0.48 | Signal components | ||||
| NT5E | 0.51 | Xanthosine/Cytidine | ||||
| Control VS Type 1 Diabetes | Human pancreas | Upregulated gene | GSE72492 | -- | ||
| ALLC | 1.61 | Urea | ||||
| ARG1 | 2.21 | Urea | ||||
| GLYAT | 1.60 | Hippuric acid | ||||
| GUCA2A | 1.95 | Guanilin | ||||
| NPY | 2.53 | Neuropeptide Y | ||||
| NT5C1A | 1.81 | Uridine/Xanthosine/Cytidine | ||||
| PCSK1 | 4.39 | Atrial natriuretic peptide | ||||
| PON1 | 1.76 | Hydroquinone | ||||
| TYMP | 1.82 | Thymine | ||||
| Downregulated gene | ||||||
| AHCY | 0.64 | Homocysteine | ||||
| ALDH2 | 0.70 | Indole-3-acetate | ||||
| AOX1 | 0.64 | N1-Methyl-2-pyridone-5-carboxamide | ||||
| BHMT2 | 0.44 | Dimethylglycine | ||||
| DPYD | 0.67 | Thymine/Uracil | ||||
| ECE1 | 0.73 | Endothelin | ||||
| FGF2 | 0.37 | Basic fibroblast growth factor | ||||
| GDE1 | 0.72 | Myoinositol | ||||
| NT5C | 0.74 | Cytidine/Inosine | ||||
| NT5C3 | 0.53 | Cytidine | ||||
| NT5E | 0.52 | Uridine/Inosine/Cytidine | ||||
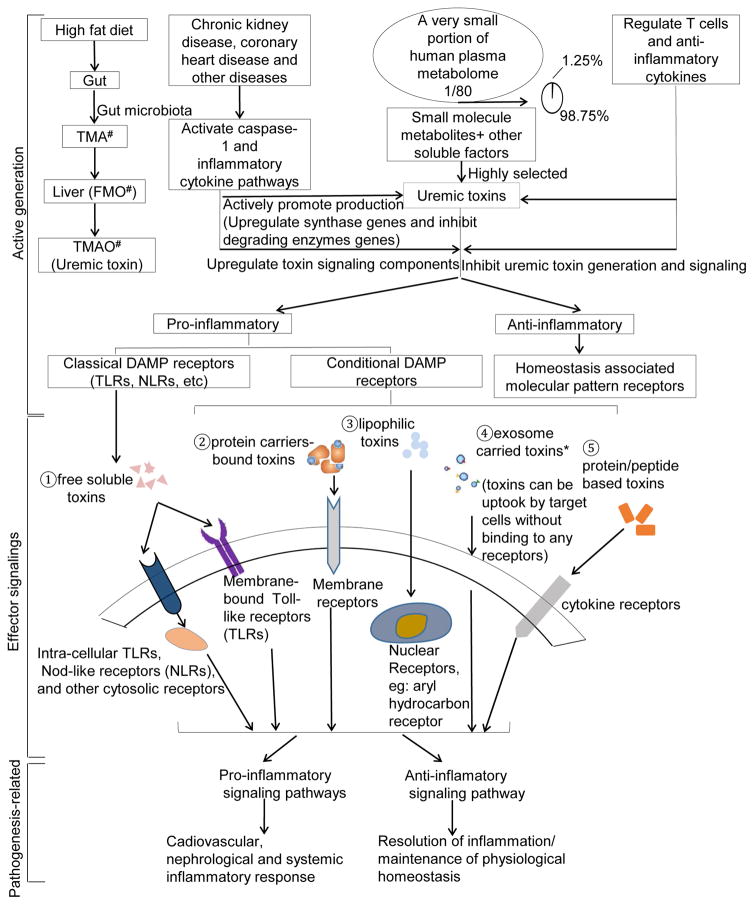
4.4. The expressions of uremic toxin genes are modulated by cytokine pathways and regulatory T cells
Our above results indicated that the upregulation of UT-generating enzymes, protein-bound UT receptors and their signaling components, and convertases for protein/peptide-based UTs in CKD-related diseases at least partially contribute to increased concentrations of UTs. The mechanisms underlying this phenomenon are unknown. We hypothesize that elevated UTs in CKD can be sensed by classical DAMP receptor pathways (27, 54). To test this hypothesis, we examined whether the expression of UT genes can be modulated in Toll-like receptor (TLR) pathways. The results showed, in Table 11, that deficiencies of TLR2, TLR3 and TLR4 resulted in decreased expression of a number of genes (3 for TLR2 deficient (TLR2−/−) mice, 4 for TLR3−/− mice and 2 for TLR4−/− mice) as well as increased expression of genes (3 for TLR2−/− mice, 6 for TLR3−/− mice and 4 for TLR4−/− mice). In addition, we also examined a new hypothesis that the expression of UT genes can be modulated via caspase-1-dependent pathways since our reports showed that caspase-1 inflammasome pathways serve as a critical sensor to bridge the risk factors for cardiovascular diseases and initiation of vascular inflammation and atherosclerosis (2, 10, 17, 29, 30). As shown in Table 12, in caspase-1 knockout mice, 12 UT genes were downregulated and 5 UT genes were upregulated, suggesting that caspase-1 pathway plays an important role in promoting UT gene expression, much more than TLR pathways. Moreover, we examined another hypothesis that the expression of UT genes can be modulated by pro-inflammatory cytokines tumor necrosis factor-α (TNF-α), IL-1β, and interferon-γ (IFN-γ) pathways. As shown in Table 13, in TNF-α-treated cells, 13 UT genes were upregulated, and 17 UT genes were downregulated; in IL-1β-treated cells, 2 UT genes were upregulated, and 3 UT genes were downregulated; and in IFN-γ-treated cells, 2 UT genes were upregulated and one UT gene was downregulated. The results suggest that first, caspase-1 pathway plays a more important role in promoting UT gene expression in comparison to other innate immune sensors DAMP receptors; and second, TNF-α pathway plays a more significant role in promoting the expression of UT genes in comparison to other pro-inflammatory cytokine pathways.
Table 11.
DAMPRs/HAMPRs signaling interactions: TLR signaling regulates the expressions of the genes encoding uremic toxins, receptor components and toxin-protein conjugation enzymes
| Genotype | Tissue | Gene | Fold Change | Toxin | GEO Database ID | PMID |
|---|---|---|---|---|---|---|
| Control VS TLR2−/− | Mus musculus colonic mucosal | Upregulated gene | GSE21845 | 21228220 | ||
| FMO3 | 1.42 | Trimethylamine | ||||
| GAMT | 1.16 | Creatine | ||||
| PON2 | 1.14 | Hydroquinone | ||||
| GLYAT | 1.19 | Hippuric acid | ||||
| Downregulated gene | ||||||
| PAOX | 0.82 | Putrescine/Spermidine | ||||
| IMPAD1 | 0.85 | Myoinositol | ||||
| RhoA | 0.81 | Signal components | ||||
| Control VS TLR3−/− | Mus musculus liver | Upregulated gene | GSE14719 | -- | ||
| ALDH9A1 | 1.14 | Indole-3-acetate | ||||
| AGMAT | 1.22 | Urea | ||||
| ALDH2 | 1.14 | Indole-3-acetate | ||||
| FMO3 | 1.00 | Trimethylamine | ||||
| FMO4 | 1.54 | Trimethylamine | ||||
| PON1 | 1.15 | Hydroquinone | ||||
| DPYD | 1.20 | Thymine/Uracil | ||||
| AHCY | 1.12 | Homocysteine | ||||
| Downregulated gene | ||||||
| ALDH3B1 | 0.84 | Phenylacetic acid | ||||
| SMOX | 0.79 | Spermidine | ||||
| NT5C | 0.95 | Cytidine/Inosine | ||||
| GDA | 0.93 | Xanthine | ||||
| Control VS TLR4−/− | Mus musculus kidney | Upregulated gene | GSE34351 | 22895517 | ||
| AGMAT | 1.45 | Urea | ||||
| ALDH3A2 | 1.27 | Indole-3-acetate | ||||
| DPYD | 1.22 | Thymine/Uracil | ||||
| ALDH2 | 1.28 | Indole-3-acetate | ||||
| Downregulated gene | ||||||
| GATM | 0.75 | Creatine | ||||
| ALDH1A3 | 0.57 | Phenylacetic acid | ||||
Table 12.
The expressions of 17 out of 169 uremic toxin genes are modulated by caspase-1 signal pathways
| Gene symbol | Fold change (Caspase-1 KO/WT) | Toxin |
|---|---|---|
| Upregulated genes (5) | ||
| XDH | 1.22 | Uric acid/Xanthine |
| CST3 | 1.25 | Cystatin C |
| FMO1 | 1.32 | Trimethylamine |
| FMO3 | 1.48 | Trimethylamine |
| LEP | 2.9 | Leptin |
| Downregulated genes (12) | ||
| IL1B | 0.47 | IL-1β |
| ARG2 | 0.62 | Urea |
| GDA | 0.67 | Xanthine |
| NT5C3 | 0.68 | Cytidine |
| UPRT | 0.69 | Uracil |
| GATM | 0.70 | γ-Guanidinobutyrate |
| NT5E | 0.73 | Cytidine/Inosine/Uridine/Xanthosine |
| ALDH1A3 | 0.79 | Phenylacetic acid |
| ALDH3B1 | 0.79 | Phenylacetic acid |
| UPP1 | 0.83 | Uracil |
| ALDH9A1 | 0.85 | Indole-3-acetate |
| RhoA | 0.89 | Protein binding uremic toxins signal components |
Table 13.
Pro-inflammation cytokine pathways regulate the expressions of the genes encoding uremic toxins, receptor components and toxin-protein conjugation enzymes.
| Treatment | Cell type | Gene | Fold Change | Toxin | GEO Dataset ID | PMID |
|---|---|---|---|---|---|---|
| Control VS TNF-α | Annulus disc cells | Upregulated gene | GSE41883 | -- | ||
| SMOX | 6.73 | Spermidine | ||||
| AKR1B1 | 4.66 | Arabinitol | ||||
| TYMP | 5.70 | Thymine | ||||
| PON2 | 2.06 | Hydroquinone | ||||
| NT5E | 2.31 | Uridine/Xanthosine/Inosine/Cytidine | ||||
| ALDH1B1 | 2.33 | Indole-3-acetate | ||||
| UPP1 | 4.59 | Uracil | ||||
| IMPAD1 | 1.89 | Myoinositol | ||||
| GLA | 1.45 | Sorbitol/Myoinositol | ||||
| ASMT | 1.05 | Melatonin | ||||
| GDA | 1.04 | Xanthine | ||||
| Myc | 1.75 | Signal components | ||||
| MDM2 | 1.05 | Signal components | ||||
| Downregulated gene | ||||||
| ALDH7A1 | 0.45 | Indole-3-acetate | ||||
| DPYD | 0.43 | Thymine/Uracil | ||||
| NT5C2 | 0.57 | Cytidine/Inosine/Xanthosine | ||||
| ALDH3A2 | 0.30 | Indole-3-acetate | ||||
| ADA | 0.43 | Inosine | ||||
| GAMT | 0.49 | Creatine | ||||
| NT5C | 0.76 | Cytidine/Inosine | ||||
| HPRT1 | 0.68 | Xanthine/Hypoxanthine | ||||
| AHCY | 0.73 | Homocysteine | ||||
| IMPA2 | 0.37 | Myoinositol | ||||
| GDE1 | 0.62 | Myoinositol | ||||
| SRM | 0.75 | Spermidine/Spermine | ||||
| CECR1 | 0.39 | Inosine | ||||
| ALDH9A1 | 0.62 | Indole-3-acetate | ||||
| RhoA | 0.68 | Signal components | ||||
| Max | 0.82 | Signal components | ||||
| CDC42 | 0.77 | Signal components | ||||
| Control VS IL-1β | Human epithelial pancreatic Mia Paca-2 cells | Upregulated gene | GSE26702 | 22313544 | ||
| ALDH2 | 5.21 | Indole-3-acetate | ||||
| AKR1B1 | 1.44 | Arabinitol | ||||
| Downregulated gene | ||||||
| ARG2 | 0.44 | Urea | ||||
| FMO5 | 0.15 | Trimethylamine | ||||
| NT5C1B | 0.19 | Cytidine/Inosine/Uridine | ||||
| ALDH3A2 | 0.64 | Indole-3-acetate | ||||
| Control VS IFN-γ | Human hepatocyte | Upregulated gene | GSE38147 | 22677194 | ||
| FMO4 | 1.63 | Trimethylamine | ||||
| GLYAT | 5.07 | Hippuric acid | ||||
| Max | 1.37 | Signal components | ||||
| Downregulated gene | ||||||
| SMOX | 0.52 | Spermidine | ||||
Finally, we wanted to examine a new hypothesis that CD4+Foxp3+ regulatory T cells (Tregs), one of the well-characterized immune tolerance cells, since we and others reported that Tregs play a critical role in suppressing vascular inflammation (13– 15); and that Tregs are weakened and expanded poorly in CKD patients in hemodialysis (55). As shown in Table 14, in Tregs versus T effector cells, 11 UT genes were upregulated; and 21 UT genes were downregulated. These results suggest that immune suppression mechanism plays an important role in inhibiting the expression of UT genes.
Table 14.
The expressions of 34 out of 169 uremic toxin genes are modulated in CD4+Foxp3+ regulatory T cells versus in T effector cells
| Gene symbol | Fold change | Toxin |
|---|---|---|
| Upregulated genes (5) | ||
| Dpyd | 1.18 | Thymine |
| Guca2b | 1.19 | Guanilin |
| Arg1 | 1.22 | Urea |
| Npy | 1.26 | Neuropeptide Y |
| Casp1 | 1.30 | Interleukin-1β/IL-18 |
| Aldh3a2 | 1.33 | Indole-3-acetate |
| Adm | 1.35 | Adrenomedullin |
| Furin | 1.48 | Adiponectin/Orexin A |
| Pon3 | 1.52 | Hydroquinone |
| Ahcyl2 | 2.29 | Homocysteine |
| Nt5e | 6.35 | Xanthosine/Cytidine |
| Downregulated genes (12) | ||
| Aldh2 | 0.29 | Indole-3-acetate |
| Fgf2 | 0.41 | Basic fibroblast growth factor |
| Impa2 | 0.45 | Myoinositol |
| Umps | 0.55 | Orotic acid |
| Ahcy | 0.58 | Homocysteine |
| Xdh | 0.58 | Uric acid |
| Gla | 0.64 | Myoinositol/Sorbitol |
| Aldh7a1 | 0.64 | Indole-3-acetate |
| Pnp | 0.67 | Hypoxanthine/Uracil/Xanthine |
| Paox | 0.69 | Putrescine |
| Nt5c2 | 0.69 | Cytidine/Inosine/Xanthosine |
| Gde1 | 0.69 | Myoinositol |
| Nt5c3 | 0.71 | Cytidine |
| Impad1 | 0.72 | Myoinositol |
| Aldh9a1 | 0.73 | Indole-3-acetate |
| Mpst | 0.77 | Thiocyanate |
| Ece1 | 0.79 | Endothelin |
| Ada | 0.80 | Inosine |
| Impa1 | 0.81 | Myoinositol |
| Ahcyl1 | 0.81 | Homocysteine |
| Dhodh | 0.84 | Orotic acid |
5. DISCUSSION
As technology, including chromatographic methods (ion exchange chromatography, gas chromatography, HPLC), spectrophotometry, fluorometry, chemiluminescence, nephelometry, radioimmunometry, nuclear magnetic resonance and mass spectrometry, has improved, more UTs have been identified (56, 57). This therefore allows for newly identified substances to be added to the list of the European Uremic Toxin (EUTox) Work Group on an ongoing basis, which provides an increasingly complex scenario on their toxicity (56). It has been well documented that some protein/peptide-based UTs, including pro-inflammatory cytokines such as TNF-α, IL-1β, IL-18, and IFN-γ, promote vascular inflammation and other organ inflammation (56). However, the issue of whether host innate immune system uses classical DAMP receptors to sense the elevation of all of other water-soluble and protein-bound UTs remains unknown. It is biochemically difficult for a few classical DAMP receptors, such as TLRs and NLRs, to bind with high affinity to all of those UTs and initiate inflammation efficiently, considering that reduced expression of TLR4 is found in uremic patients (58).
To solve this problem, here, similar to what we reported recently for lysophospholipids, we examined our new hypothesis that UTs can serve as conditional pro-inflammatory DAMPs or anti-inflammatory HAMPs, and that UTs use classical DAMP receptors as well as their intrinsic receptors including RAGE, and serum albumin-toxin receptors to modulate inflammation (54). We have made the following new findings: 1) Chronic kidney disease selectively accumulates a very small fraction of human serum small-molecule metabolome, roughly 1/80th, as UTs, suggesting that elevation of UTs is highly specific, and may not all result from dysfunctional glomerular filtration; 2) The serum concentrations of the majority of UTs are increased not only in CKD but also in other diseases, suggesting that some so-called UTs can also be increased when patients have no renal failure; 3) Protein-bound UTs either induce or suppress the expression of pro-inflammatory molecules rather than only promoting inflammation; 4) The expression of UT genes is modulated in the proximal tubules of patients with CKD, and adipose tissue of patients with coronary artery disease (CAD), more than in patients with metabolic syndrome and type 2 diabetes, pointing out the potential mechanisms underlying the roles of UTs in accelerating CAD more than other diseases in patients with CKD; 5) The expression of UT genes is upregulated by caspase-1-dependent pathway and pro-inflammatory cytokine TNF-α pathways, more than other innate immune sensors, such as TLR pathways, and IL-1β and IFN-γ pathways. This suggests that caspase-1 pathway and TNF-α pathways are potential points of focus for the future development of novel therapy in suppressing UT accelerated pathologies; and 6) The expression of UT genes is inhibited in Tregs, which emphasizes the importance of Tregs in suppressing the pathogenic effects of UTs (55).
Since 1967, dialysis has been used a standard of care for patients with end-stage renal disease, with numerous new methods being used to complement the dialysis care (59, 60). Dialysis is based on a classical hypothesis that passive accumulation of UTs is due to decreased glomerular filtration in CKD. However, our new findings revealed that UTs represent only 1/80th of human serum small-molecule metabolome, showing that UT accumulation is selective. In addition, considering that roughly 10% of the total metabolites have been identified in human serum metabolome (http://www.serummetabolome.ca), we can further postulate that actually CKD highly selectively accumulates a very tiny fraction of human serum small-molecule metabolome, roughly 1/800th, as UTs (44). Moreover, our results showed that the serum concentrations of the majority of UTs are increased not only in CKD but also in other diseases. Our results suggest that novel anti-caspase-1 and anti-TNF-α therapies and therapeutics in controlling UT-increased diseases together with dialysis could be developed.
Protein-bound UTs are poorly removed by current dialysis techniques because their size is larger than the pore size of dialysis membrane (61). These protein-bound UTs, such as indoxyl sulfate, can induce upregulation of endothelial adhesion molecules, the hallmarks of endothelial cell activation, by binding to human aryl hydrocarbon receptor (AhR) to activate NF-kB and mitogen-activated protein kinases (MAPKs), and NADPH oxidase to increase reactive oxygen species (ROS), both cytosolic ROS and mitochondrial ROS as we recently reported (3, 61, 62). In addition, many UTs bind specifically to the Sudlow’s sites I and II of human serum albumin mainly via electrostatic and/or van der Waals forces (63–66). Five types of serum albumin receptors have been identified, including glycoproteins Gp60, Gp30 and Gp18, SPARC, the megalin/cubilin complex, RAGE and the neonatal Fc receptor (FcRn) (51). Moreover, advanced glycation end products (AGE) in UTs can also use RAGE to trigger various intracellular events, such as oxidative stress and inflammation, leading to cardiovascular complications (67, 68). Taken together, our findings suggest that protein-bound UTs may not necessarily use classical DAMP receptors such as TLRs and NLRs to initiate inflammation, may use their intrinsic receptors including AhR, several serum albumin receptors and RAGE to promote inflammation. This conclusion supports our new classification of UTs as conditional danger-associated molecular patterns (DAMPs) or homeostasis-associated molecular patterns (HAMPs). The significance for classifying UTs as conditional DAMPs and HAMPs is that, this model will guide our future work of examining the pathways of new conditional DAMP receptors and HAMP receptors for novel therapeutic purposes.
Recent significant reports and reviews demonstrated a proof of principle that choline, derived by food (dietary) intake from intestine, requires intestinal bacterial enzyme-dependent transformation into trimethylamine (TMA), which is further absorbed into the blood circulation and is transformed into trimethylamine N-oxide (TMAO) in host liver by flavin containing monooxygenases (FMOs) (69–71). TMAO is a newly characterized UT that exhibits genetic and dietary regulation, and promotes CKD, cardiovascular disease, impaired glucose intolerance, and atherosclerosis (72–77). We found that the expression of FMO1-5 is modulated by inflammatory pathways, including caspase-1 and TLR pathways. This new finding regarding TMAO generation pathway, together with other results presented in this study as well as other reports, allows us to propose a new working model (Figure 6), which is summarized in the following points of view: First, rather than passive accumulation of endogenous metabolites, a very small fraction of human metabolome, roughly 1/80th of human plasma metabolome, or 1/800th of total human metabolome, eventually becomes selected to be UTs, suggesting that a highly selective mechanism is underlying the generation of UTs; Second, the expression of some UT synthases and signaling genes is significantly increased in patients with CKD, CAD and other diseases, suggesting that an increase in UTs; Third, the proof of principle demonstrated in TMAO pathway suggests that several factors, including diet, intestinal microbiome, as well as FMOs in host liver all contribute to microbiome-generated UTs; Fourth, regulatory T cells and anti-inflammatory cytokines may inhibit the gene expression of UT synthases and signaling pathway components; and Fifth, UTs serve as conditional DAMPs and HAMPs, whose intrinsic receptors, in addition to TLRs and NLRs, may initiate UT signaling for regulating vascular inflammation and other inflammatory diseases. These new findings have significantly improved our understanding of molecular mechanisms underlying the roles of UTs in accelerating vascular inflammation, and UT generation, which provide novel insights for the future development of new therapeutics for CKD and CKD-promoted cardiovascular disease and other diseases.
Figure 6.
Our new working model: the generations of pathologically uremic toxins can be increased in chromic kidney disease and other inflammatory diseases rather than purely passive accumulation due to failing kidney function. Uremic toxins are conditional danger associated molecular patterns (DAMPs) or homeostasis associated molecular patterns (HAMPs), which are functional in multiple modes in modulating inflammation. The detailed descriptions of this new working model were presented in the Conclusion section of this paper. # TMA: trimethylamine; FMO: Flavin-containing monooxygenase; TMAO: Trimethylamine N-oxide; TLRs: Toll-like receptors; NLRs: Nod-like receptors. Adapted with permission from (Ref number 25143819, 24599232).
6. CONCLUSIONS
Our new findings and others’ recent reports allow us to propose a new working model (Figure 6), which is summarized in the following points of view: First, rather than passive accumulation of endogenous metabolites, a very small fraction of human metabolome, roughly 1/80th of human plasma metabolome, or 1/800th of total human metabolome, eventually becomes selected to be UTs, suggesting that a highly selective mechanism is underlying the generation of UTs; Second, the expression of some UT synthases and signaling genes is significantly increased in patients with CKD, CAD and other diseases, suggesting that an increase in UTs; Third, the proof of principle demonstrated in TMAO pathway suggests that several factors, including diet, intestinal microbiome, as well as FMOs in host liver all contribute to microbiome-generated UTs; Fourth, regulatory T cells and anti-inflammatory cytokines may inhibit the gene expression of UT synthases and signaling pathway components; and Fifth, UTs serve as conditional DAMPs and HAMPs, whose intrinsic receptors, in addition to TLRs and NLRs, may initiate UT signaling for regulating vascular inflammation and other inflammatory diseases. These new findings have significantly improved our understanding of molecular mechanisms underlying the roles of UTs in accelerating vascular inflammation, and UT generation, which provide novel insights for the future development of new therapeutics for CKD and CKD-promoted cardiovascular disease and other diseases.
Acknowledgments
This work is partially supported by NIH grants to Drs. XF. Yang, H. Wang and ET. Choi and the Chinese National Nature Science Foundation Grants (Award number 81570626 and 81450033) to Dr. Li.RS carried out the data gathering, data analysis and prepared tables and figures. CJ, JZ, LQW, YFL, GN, HFF, YS, CS, WYY, YFL, XW, ETC, RSL, HW aided with analysis of the data. XFY supervised the experimental design, data analysis, and manuscript writing. All authors read and approved the final manuscript.
Abbreviations
- DAMP
danger signal-associated molecular patterns
- HAMP
homeostasis-associated molecular patterns
- CKD
chronic kidney disease
- UT
uremic toxins
- CAD
coronary artery disease
- TNF-α
Tumor necrosis factor α
- TLR
toll-like receptors
- IL-1β
Interleukin-1 beta
- IFN-γ
Interferon-gamma
- EC
endothelial cell
- ESRD
end-stage renal disease
- GFR
glomerular filtration rate
- BUN
blood urea nitrogen
- cLDL
carbamylated LDL
- VSMC
vascular smooth muscle cell
- PAMP
pathogen-associated molecular patterns
- NLR
NOD (nucleotide binding and oligomerization domain)-like receptors
- RAGE
advanced glycation end products
- MCP-1
monocyte chemoattractant protein-1
- A2AR
adenosine A2A receptor
- A2R
adenosine A2 receptor
- IL-18R
interleukin-18 receptor
- IL6R
interleukin 6 receptor
- IL-1R
Interleukin-1 receptor
- LEPR/OBR
Leptin receptor
- MT1/MT2
Melatonin receptor
- RZR/ROR
orphan receptor
- RAGE
receptor for advanced glycation endproduct
- TNFR
tumor necrosis factor receptor
- AHR
Aryl hydrocarbon receptor
- GPR35
G protein-coupled receptor 35
- GP85/CD44
CD44 antigen
- Octβ2R
the beta adrenergic-like octopamine receptor
- GPCR
G-protein-coupled receptors
- NK-1R, NK-2R, NK-3R
Neurokinin-1 receptor, Neurokinin-2 receptor, Neurokinin-3 receptor
- CRLR
calcitonin receptor-like receptor
- CALCRL
Calcitonin receptor-like
- RAMP1
Receptor activity modifying protein 1
- GHSR1a
Growth hormone secretagogue receptor
- OX1R/OX2R
Orexin receptor type 1, Orexin receptor type 2
- PTH1R
parathyroid hormone 1 receptor
- GC-C, GC-D
receptor-guanylate cyclase
- VPAC1, VPAC2
vasoactive intestinal peptide (VIP) receptor
- AdipoR1, AdipoR2
Adiponectin receptor
- NPR1, NPR2, NPR3
Atrial natriuretic peptide receptor
- bFGF-R1, bFGF-R2
basic fibroblast growth factor receptors
- CCK1R, CCK2R
cholecystokinin receptor
- ETA, ETB1, ETB2, ETC
endothelin receptors
- NPY1R, NPY2R, NPY3R, NPY4R, NPY5R, NPY6R
Neuropeptide Y receptors
References
- 1.Reikes ST. Trends in end-stage renal disease. Epidemiology, morbidity, and mortality. Postgrad Med J. 2000;108:124–126. 129–131, 135–126. doi: 10.3810/pgm.2000.07.1152. [DOI] [PubMed] [Google Scholar]
- 2.Yin Y, Li X, Sha X, Xi H, Li YF, Shao Y, Mai J, Virtue A, Lopez-Pastrana J, Meng S, Tilley DG, Monroy MA, Choi ET, Thomas CJ, Jiang X, Wang H, Yang XF. Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arterioscler Thromb Vasc Biol. 2015;35:804–816. doi: 10.1161/ATVBAHA.115.305282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Fang P, Li Y, Kuo YM, Andrews AJ, Nanayakkara G, Johnson C, Fu H, Shan H, Du F, Hoffman NE, Yu D, Eguchi S, Madesh M, Koch WJ, Sun J, Jiang X, Wang H, Yang X. Mitochondrial reactive oxygen species mediate lysophosphatidylcholine-induced endothelial cell activation. Arterioscler Thromb Vasc Biol. 2016;36:1090–1100. doi: 10.1161/ATVBAHA.115.306964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang P, Zhang D, Cheng Z, Yan C, Jiang X, Kruger WD, Meng S, Arning E, Bottiglieri T, Choi ET, Han Y, Yang XF, Wang H. Hyperhomocysteinemia potentiates hyperglycemia-induced inflammatory monocyte differentiation and atherosclerosis. Diabetes. 2014;63:4275–4290. doi: 10.2337/db14-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xi H, Zhang Y, Xu Y, Yang WY, Jiang X, Sha X, Cheng X, Wang J, Qin X, Yu J, Ji Y, Yang X, Wang H. Caspase-1 inflammasome activation mediates homocysteine-induced pyrop-apoptosis in endothelial cells. Circulation research. 2016;118:1525–1539. doi: 10.1161/CIRCRESAHA.116.308501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrer LM, Monroy AM, Lopez-Pastrana J, Nanayakkara G, Cueto R, Li YF, Li X, Wang H, Yang XF, Choi ET. Caspase-1 plays a critical role in accelerating chronic kidney disease-promoted neointimal hyperplasia in the carotid artery. J Cardiovasc Transl Res. 2016;9:135–144. doi: 10.1007/s12265-016-9683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monroy MA, Fang J, Li S, Ferrer L, Birkenbach MP, Lee IJ, Wang H, Yang XF, Choi ET. Chronic kidney disease alters vascular smooth muscle cell phenotype. Front Biosci (Landmark Ed) 2015;20:784–795. doi: 10.2741/4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sha X, Meng S, Li X, Xi H, Maddaloni M, Pascual DW, Shan H, Jiang X, Wang H, Yang XF. Interleukin-35 inhibits endothelial cell activation by suppressing mapk-ap-1 pathway. J Biol Chem. 2015;290:19307–19318. doi: 10.1074/jbc.M115.663286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao Y, Cheng Z, Li X, Chernaya V, Wang H, Yang XF. Immunosuppressive/anti-inflammatory cytokines directly and indirectly inhibit endothelial dysfunction--a novel mechanism for maintaining vascular function. J Hematol Oncol. 2014;7:80. doi: 10.1186/s13045-014-0080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Pastrana J, Ferrer LM, Li YF, Xiong X, Xi H, Cueto R, Nelson J, Sha X, Li X, Cannella AL, Imoukhuede PI, Qin X, Choi ET, Wang H, Yang XF. Inhibition of caspase-1 activation in endothelial cells improves angiogenesis: A novel therapeutic potential for ischemia. J Biol Chem. 2015;290:17485–17494. doi: 10.1074/jbc.M115.641191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang CE, Wei W, Liu YH, Peng JH, Tian Q, Liu GP, Zhang Y, Wang JZ. Hyperhomocysteinemia increases beta-amyloid by enhancing expression of gamma-secretase and phosphorylation of amyloid precursor protein in rat brain. J Pathol. 2009;174:1481–1491. doi: 10.2353/ajpath.2009.081036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D, Fang P, Jiang X, Nelson J, Moore JK, Kruger WD, Berretta RM, Houser SR, Yang X, Wang H. Severe hyperhomocysteinemia promotes bone marrow-derived and resident inflammatory monocyte differentiation and atherosclerosis in ldlr/cbs-deficient mice. Circulation research. 2012;111:37–49. doi: 10.1161/CIRCRESAHA.112.269472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiong Z, Song J, Yan Y, Huang Y, Cowan A, Wang H, Yang XF. Higher expression of bax in regulatory t cells increases vascular inflammation. Front Biosci. 2008;13:7143–7155. doi: 10.2741/3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong Z, Yan Y, Song J, Fang P, Yin Y, Yang Y, Cowan A, Wang H, Yang XF. Expression of tctp antisense in cd25 (high) regulatory t cells aggravates cuff-injured vascular inflammation. Atherosclerosis. 2009;203:401–408. doi: 10.1016/j.atherosclerosis.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang WY, Shao Y, Lopez-Pastrana J, Mai J, Wang H, Yang XF. Pathological conditions re-shape physiological tregs into pathological tregs. Burns & trauma. 2015;3:1. doi: 10.1186/s41038-015-0001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson J, Wu Y, Jiang X, Berretta R, Houser S, Choi E, Wang J, Huang J, Yang X, Wang H. Hyperhomocysteinemia suppresses bone marrow cd34+/vegf receptor 2+ cells and inhibits progenitor cell mobilization and homing to injured vasculature-a role of beta1-integrin in progenitor cell migration and adhesion. FASEB J. 2015;29:3085–3099. doi: 10.1096/fj.14-267989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li YF, Huang X, Li X, Gong R, Yin Y, Nelson J, Gao E, Zhang H, Hoffman NE, Houser SR, Madesh M, Tilley DG, Choi ET, Jiang X, Huang CX, Wang H, Yang XF. Caspase-1 mediates hyperlipidemia-weakened progenitor cell vessel repair. Front Biosci (Landmark Ed) 2016;21:178–191. doi: 10.2741/4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao Y, Chernaya V, Johnson C, Yang WY, Cueto R, Sha X, Zhang Y, Qin X, Sun J, Choi ET, Wang H, Yang XF. Metabolic diseases downregulate the majority of histone modification enzymes, making a few upregulated enzymes novel therapeutic targets-“sand out and gold stays”. J Cardiovasc Transl Res. 2016;9:49–66. doi: 10.1007/s12265-015-9664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahn MR, Robbins MJ, Kim MC, Fuster V. Management of cardiovascular disease in patients with kidney disease. Nature reviews Cardiology. 2013;10:261–273. doi: 10.1038/nrcardio.2013.15. [DOI] [PubMed] [Google Scholar]
- 20.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: Effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 21.Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560–567. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 22.Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med. 2008;18:228–232. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moradi H, Sica DA, Kalantar-Zadeh K. Cardiovascular burden associated with uremic toxins in patients with chronic kidney disease. Am J Nephrol. 2013;38:136–148. doi: 10.1159/000351758. [DOI] [PubMed] [Google Scholar]
- 24.Apostolov EO, Basnakian AG, Ok E, Shah SV. Carbamylated low-density lipoprotein: Nontraditional risk factor for cardiovascular events in patients with chronic kidney disease. J Ren Nutr. 2012;22:134–138. doi: 10.1053/j.jrn.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Speer T, Owala FO, Holy EW, Zewinger S, Frenzel FL, Stahli BE, Razavi M, Triem S, Cvija H, Rohrer L, Seiler S, Heine GH, Jankowski V, Jankowski J, Camici GG, Akhmedov A, Fliser D, Luscher TF, Tanner FC. Carbamylated low-density lipoprotein induces endothelial dysfunction. Eur Heart J. 2014;35:3021–3. doi: 10.1093/eurheartj/ehu111. [DOI] [PubMed] [Google Scholar]
- 26.Mehta JL, Basnakian AG. Interaction of carbamylated ldl with lox-1 in the induction of endothelial dysfunction and atherosclerosis. Eur Heart J. 2014;35:2996–7. doi: 10.1093/eurheartj/ehu122. [DOI] [PubMed] [Google Scholar]
- 27.Yang XF, Yin Y, Wang H. Vascular inflammation and atherogenesis are activated via receptors for pamps and suppressed by regulatory t cells. Drug Discov Today Ther Strateg. 2008;5:125–142. doi: 10.1016/j.ddstr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venereau E, Ceriotti C, Bianchi ME. Damps from cell death to new life. Front Immunol. 2015;6:422. doi: 10.3389/fimmu.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin Y, Pastrana JL, Li X, Huang X, Mallilankaraman K, Choi ET, Madesh M, Wang H, Yang XF. Inflammasomes: Sensors of metabolic stresses for vascular inflammation. Front Biosci (Landmark Ed) 2013;18:638–649. doi: 10.2741/4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin Y, Yan Y, Jiang X, Mai J, Chen NC, Wang H, Yang XF. Inflammasomes are differentially expressed in cardiovascular and other tissues. Int J Immunopathol Pharmacol. 2009;22:311–322. doi: 10.1177/039463200902200208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Fu H, Nanayakkara G, Li Y, Shao Y, Johnson C, Cheng J, Yang WY, Yang F, Lavallee M, Xu Y, Cheng X, Xi H, Yi J, Yu J, Choi ET, Wang H, Yang X. Novel extracellular and nuclear caspase-1 and inflammasomes propagate inflammation and regulate gene expression: A comprehensive database mining study. J Hematol Oncol. 2016;9:122. doi: 10.1186/s13045-016-0351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin Y, Pastrana JL, Li X, Huang X, Mallilankaraman K, Choi ET, Madesh M, Wang H, Yang XF. Inflammasomes: Sensors of metabolic stresses for vascular inflammation. Front Biosci. 2013;18:638–649. doi: 10.2741/4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Li YF, Nanayakkara G, Shao Y, Liang B, Cole L, Yang WY, Li X, Cueto R, Yu J, Wang H, Yang XF. Lysophospholipid receptors, as novel conditional danger receptors and homeostatic receptors modulate inflammation-novel paradigm and therapeutic potential. J Cardiovasc Transl Res. 2016;9:343–359. doi: 10.1007/s12265-016-9700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang X, Gong R, Li X, Virtue A, Yang F, Yang IH, Tran AH, Yang XF, Wang H. Identification of novel pretranslational regulatory mechanisms for nf-kappab activation. J Biol Chem. 2013;288:15628–15640. doi: 10.1074/jbc.M113.460626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neirynck N, Vanholder R, Schepers E, Eloot S, Pletinck A, Glorieux G. An update on uremic toxins. Int Urol Nephrol. 2013;45:139–150. doi: 10.1007/s11255-012-0258-1. [DOI] [PubMed] [Google Scholar]
- 36.Niwa T. Update of uremic toxin research by mass spectrometry. Mass Spectrom Rev. 2011;30:510–521. doi: 10.1002/mas.20323. [DOI] [PubMed] [Google Scholar]
- 37.Vanholder R, Glorieux G, De Smet R, Lameire N. New insights in uremic toxins. Kidney Int Suppl. 2013:S6–10. doi: 10.1046/j.1523-1755.63.s84.43.x. [DOI] [PubMed] [Google Scholar]
- 38.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koyama Y, Takeishi Y, Arimoto T, Niizeki T, Shishido T, Takahashi H, Nozaki N, Hirono O, Tsunoda Y, Nitobe J, Watanabe T, Kubota I. High serum level of pentosidine, an advanced glycation end product (age), is a risk factor of patients with heart failure. J Card Fail. 2017;13:199–206. doi: 10.1016/j.cardfail.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Virtue A, Wang H, Yang XF. Micrornas and toll-like receptor/interleukin-1 receptor signaling. J Hematol Oncol. 2012;5:66. doi: 10.1186/1756-8722-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sladek FM. What are nuclear receptor ligands? Mol Cell Endocrinol. 2011;334:3–13. doi: 10.1016/j.mce.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Velenosi TJ, Feere DA, Sohi G, Hardy DB, Urquhart BL. Decreased nuclear receptor activity and epigenetic modulation associates with down-regulation of hepatic drug-metabolizing enzymes in chronic kidney disease. FASEB J. 2014;28:5388–5397. doi: 10.1096/fj.14-258780. [DOI] [PubMed] [Google Scholar]
- 43.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 44.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS. The human serum metabolome. PloS one. 2011;6:e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyamoto Y, Iwao Y, Mera K, Watanabe H, Kadowaki D, Ishima Y, Chuang VT, Sato K, Otagiri M, Maruyama T. A uremic toxin, 3-carboxy-4-methyl-5-propyl-2-furanpropionate induces cell damage to proximal tubular cells via the generation of a radical intermediate. Biochem Pharmacol. 2012;84:1207–1214. doi: 10.1016/j.bcp.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 46.Niwa T, Takeda N, Yoshizumi H. Rna metabolism in uremic patients: Accumulation of modified ribonucleosides in uremic serum. Technical note. Kidney Int. 1998;53:1801–1806. doi: 10.1046/j.1523-1755.1998.00944.x. [DOI] [PubMed] [Google Scholar]
- 47.Vanholder R, De Smet R, Lesaffer G. P-cresol: A toxin revealing many neglected but relevant aspects of uraemic toxicity. Nephrol Dial Transplant. 1999;14:2813–2815. doi: 10.1093/ndt/14.12.2813. [DOI] [PubMed] [Google Scholar]
- 48.Mandal S, Mandal A, Johansson HE, Orjalo AV, Park MH. Depletion of cellular polyamines, spermidine and spermine, causes a total arrest in translation and growth in mammalian cells. Proc Natl Acad Sci U S A. 2013;110:2169–2174. doi: 10.1073/pnas.1219002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Viaene L, Annaert P, de Loor H, Poesen R, Evenepoel P, Meijers B. Albumin is the main plasma binding protein for indoxyl sulfate and p-cresyl sulfate. Biopharm Drug Dispos. 2013;34:165–175. doi: 10.1002/bdd.1834. [DOI] [PubMed] [Google Scholar]
- 50.Merlot AM, Kalinowski DS, Richardson DR. Unraveling the mysteries of serum albumin-more than just a serum protein. Front Physiol. 2014;5:299. doi: 10.3389/fphys.2014.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larsen MT, Kuhlmann M, Hvam ML, Howard KA. Albumin-based drug delivery: Harnessing nature to cure disease. Mol Cell Ther. 2016;4:3. doi: 10.1186/s40591-016-0048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ, Lotze MT. Rage (receptor for advanced glycation endproducts), rage ligands, and their role in cancer and inflammation. J Transl Med. 2009;7:17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez-Calero L, Martin-Lorenzo M, Alvarez-Llamas G. Exosomes: A potential key target in cardio-renal syndrome. Front Immunol. 2014;5:465. doi: 10.3389/fimmu.2014.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Li YF, Nanayakkara G, Shao Y, Liang B, Cole L, Yang WY, Li X, Cueto R, Yu J, Wang H, Yang XF. Lysophospholipid receptors, as novel conditional danger receptors and homeostatic receptors modulate inflammation-novel paradigm and therapeutic potential. J Cardiovasc Transl Res. 2016;9:343–59. doi: 10.1007/s12265-016-9700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Afzali B, Edozie FC, Fazekasova H, Scotta C, Mitchell PJ, Canavan JB, Kordasti SY, Chana PS, Ellis R, Lord GM, John S, Hilton R, Lechler RI, Lombardi G. Comparison of regulatory t cells in hemodialysis patients and healthy controls: Implications for cell therapy in transplantation. Clin J Am Soc Nephro: CJASN. 2013;8:1396–1405. doi: 10.2215/CJN.12931212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lisowska-Myjak B. Uremic toxins and their effects on multiple organ systems. Nephron Clinical practice. 2014;128:303–311. doi: 10.1159/000369817. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka H, Sirich TL, Plummer NS, Weaver DS, Meyer TW. An enlarged profile of uremic solutes. PloS one. 2015;10:e0135657. doi: 10.1371/journal.pone.0135657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ando M, Shibuya A, Tsuchiya K, Akiba T, Nitta K. Reduced capacity of mononuclear cells to synthesize cytokines against an inflammatory stimulus in uremic patients. Nephron Clinical practice. 2006;104:c113–119. doi: 10.1159/000094446. [DOI] [PubMed] [Google Scholar]
- 59.Klarenbach SW, Tonelli M, Chui B, Manns BJ. Economic evaluation of dialysis therapies. Nature reviews Nephrology. 2014;10:644–652. doi: 10.1038/nrneph.2014.145. [DOI] [PubMed] [Google Scholar]
- 60.Glorieux G, Tattersall J. Uraemic toxins and new methods to control their accumulation: Game changers for the concept of dialysis adequacy. Clin Kidney J. 2015;8:353–362. doi: 10.1093/ckj/sfv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ito S, Yoshida M. Protein-bound uremic toxins: New culprits of cardiovascular events in chronic kidney disease patients. Toxins. 2014;6:665–678. doi: 10.3390/toxins6020665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sallee M, Dou L, Cerini C, Poitevin S, Brunet P, Burtey S. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: A new concept to understand cardiovascular complications of chronic kidney disease. Toxins. 2014;6:934–949. doi: 10.3390/toxins6030934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sudlow G, Birkett DJ, Wade DN. The characterization of two specific drug binding sites on human serum albumin. Mol Pharmacol. 1975;11:824–832. [PubMed] [Google Scholar]
- 64.Takamura N, Maruyama T, Otagiri M. Effects of uremic toxins and fatty acids on serum protein binding of furosemide: Possible mechanism of the binding defect in uremia. Clin Chem. 1997;43:2274–2280. [PubMed] [Google Scholar]
- 65.Jourde-Chiche N, Dou L, Cerini C, Dignat-George F, Vanholder R, Brunet P. Protein-bound toxins--update 2009. Semin Dial. 2009;22:334–339. doi: 10.1111/j.1525-139X.2009.00576.x. [DOI] [PubMed] [Google Scholar]
- 66.Vanholder R, Hoefliger N, De Smet R, Ringoir S. Extraction of protein bound ligands from azotemic sera: Comparison of 12 deproteinization methods. Kidney Int. 1992;41:1707–1712. doi: 10.1038/ki.1992.244. [DOI] [PubMed] [Google Scholar]
- 67.Jourde-Chiche N, Dou L, Cerini C, Dignat-George F, Brunet P. Vascular incompetence in dialysis patients--protein-bound uremic toxins and endothelial dysfunction. Semin Dial. 2011;24:327–337. doi: 10.1111/j.1525-139X.2011.00925.x. [DOI] [PubMed] [Google Scholar]
- 68.Stinghen AE, Massy ZA, Vlassara H, Striker GE, Boullier A. Uremic toxicity of advanced glycation end products in ckd. JASN. 2016;27:354–370. doi: 10.1681/ASN.2014101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fennema D, Philips IR, Shephard EA. Trimethylamine and trimethylamine n-oxide, a flavin-containing monooxygenase 3 (fmo3)-mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab Dispos. 2016;44:1839–1850. doi: 10.1124/dmd.116.070615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aron-Wisnewsky J, Clement K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nature reviews Nephrology. 2016;12:169–181. doi: 10.1038/nrneph.2015.191. [DOI] [PubMed] [Google Scholar]
- 71.Obeid R, Awwad HM, Rabagny Y, Graeber S, Herrmann W, Geisel J. Plasma trimethylamine n-oxide concentration is associated with choline, phospholipids, and methyl metabolism. Am J Clin Nutr. 2016;103:703–711. doi: 10.3945/ajcn.115.121269. [DOI] [PubMed] [Google Scholar]
- 72.Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ. Trimethylamine-n-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine n-oxide (tmao) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circulation research. 2015;116:448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. Dietary trimethylamine n-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118:476–481. doi: 10.1016/j.jbiosc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 76.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]