Abstract
High-dose atorvastatin pretreatment was proved reducing the risk of contrast-induced acute kidney injury (CI-AKI), especially in patients with high C-reactive protein (CRP) levels. We evaluated the effects of common atorvastatin doses (double vs usual) on the risk of CI-AKI and mortality.
We recorded outcomes from 1319 patients who were administered periprocedural common doses of atorvastatin. The risks of CI-AKI and mortality between double-dose (40 mg/d) and usual-dose atorvastatin (20 mg/d) were compared using multivariable regression models in all patients or CRP tertile subgroups.
Seventy-six (5.8%) patients developed CI-AKI. Double-dose atorvastatin compared with usual-dose did not further reduce the risk of CI-AKI (adjusted odds ratio [OR]: 2.28, 95% confidence interval [CI]: 0.92–5.62, P = .074), even for patients in the highest CRP tertile (>8.33 mg/L; adjusted OR: 3.76, 95% CI: 0.83–17.05, P = .086). Similar results were observed in reducing mortality in all patients (adjusted hazard ratio: 0.47, 95% CI: 0.10–2.18; P = .339) and in the highest CRP tertiles (P = .424). In the subgroup analysis, double-dose atorvastatin increased risk of CI-AKI in patients with creatinine clearance (CrCl) < 60 mL/min, anemia, contrast volume > 200 mL and > 2 stents implanted (P = .046, .009, .024, and .026, respectively).
Daily periprocedural double-dose atorvastatin was not associated with a reduced risk of CI-AKI compared with usual-dose, and did not provide an improved long-term prognosis, even in patients with high CRP levels. However, it increased the risk of CI-AKI in patients with a high contrast volume/CrCl.
Keywords: atorvastatin, contrast-induced acute kidney injury, C-reactive protein, dose, percutaneous coronary intervention
1. Introduction
Contrast-induced acute kidney injury (CI-AKI) is a common complication of coronary angiography (CAG) or percutaneous coronary intervention (PCI), and is associated with increased mortality.[1,2] The development of CI-AKI may be associated with various inflammation-related factors, including endothelium dysfunction, oxidative stress, and renal vasoconstriction.[3,4] In addition, C-reactive protein (CRP) as a maker of systemic inflammation is associated with an increased risk of CI-AKI.[5]
Statins were reported to reduce the risk of CI-AKI by reducing oxidative stress and inflammation and improving endothelial function.[6] The ARMYDA-CIN study demonstrated that the early use of high-dose atorvastatin (120 mg) reduced CRP levels and the risk of CI-AKI, compared with a placebo treatment.[7] Similarly, Zhou et al[8] have suggested that preprocedural high-dose atorvastatin (80 mg) is superior to low-dose atorvastatin (10 mg) in reducing the risk of CI-AKI among patients who are undergoing CAG or PCI. However, in a recent randomized trial of 615 cardiac patients, investigators found that high-dose perioperative atorvastatin treatment did not reduce the risk or severity of AKI, among patients naive to statins or patients already using a statin.[9] Atorvastatin doses of 120 mg, 80 mg, and 10 mg are rarely administered in clinical practice, where usual-dose (20 mg) or double-dose (40 mg) are the most commonly used doses. However, the effects of common atorvastatin doses on the risk of CI-AKI are not well understood, especially in patients with high CRP levels.
Therefore, the current study compared the associations between inhospital short-term periprocedural double-dose and usual-dose atorvastatin and the risk of CI-AKI and patients’ outcomes after CAG or PCI.
2. Methods
2.1. Study design and patient population
This prospective observational study reviewed all consecutive patients who underwent CAG or PCI according to our institutional protocol between January 2010 and October 2012. As in a previous study[10] and in the PRECOMIN study, we included patients who were ≥18 years old, agreed to stay in the hospital for 2 to 3 days after CAG, and received short-term periprocedural atorvastatin therapy (20 or 40 mg/d) from admission (1–3 days before the procedure) until discharge (2–3 days after the procedure). Based on the updated European Society of Urogenital Radiology Contrast Media Safety Committee guidelines,[11] the exclusion criteria were pregnancy, lactation, intravascular administration of a contrast medium within the previous 7 days or 3 days postoperation (n = 83), no use of low-osmolarity contrast agents (n = 130), incomplete CRP determination and other statins used (not atorvastatin) or nonstatin treatment during hospitalization (n = 1954), cardiovascular surgery or endovascular repair (n = 382), end-stage renal disease or renal replacement (n = 7), missing preoperative or postoperative creatinine data (n = 61), malignancy (n = 3), no use of isotonic saline for hydration (n = 18), and use of drugs with potential effects on renal function (sodium bicarbonate, nonsteroid anti-inflammatory drugs, aminoglycosides, cyclosporin, and cisplatin) within 48 hours before and 72 hours after the procedure.
Based on these criteria, 1319 patients who were treated with periprocedural short-term atorvastatin were included in our analysis. The dose of atorvastatin of each patient was decided by clinicians according to the individual patients’ clinical characteristics and clinical experience with bias. The patients were then divided into the double-dose group (n = 334, 40 mg/d) and the usual-dose group (n = 985, 20 mg/d). Follow-up events were carefully monitored and recorded by trained nurses through office visits and telephone interviews at 1, 6, 12, and 24 months after the CAG. The mean follow-up duration was 2.52 ± 0.85 years (median: 2.43 years, interquartile range: 1.84–3.24 years). The study's design has been approved by our institutional ethics review board, and all patients gave their written informed consent.
2.2. Study procedures
The CAG was performed according to standard clinical practice, using standard guide catheters, guide wires, balloon catheters, and stents via the femoral or radial approaches. The PCI was performed immediately after diagnostic angiography when appropriate. Before and after CAG or PCI, all patients continued to take clopidogrel (75 mg/d for at least 12 months) and/or aspirin (100 mg/d indefinitely). The contrast dose was selected based on the interventional cardiologist's discretion, and all patients received nonionic low-osmolarity contrast agents (either Iopamiron or Ultravist, both 370 mg I/mL). All patients were treated based on the AHA/ACCF guidelines.[12] According to our institutional protocol,[10] serum creatinine concentrations were measured by Jaffe's method for all patients at hospital admission and on days 1, 2, and 3 after the CAG or PCI.
The creatinine clearance rate (CrCl) was calculated using the serum creatinine concentration and the Cockcroft-Gault formula[13] and the V/CrCl ratio was also calculated. Patients received a continuous intravenous infusion of isotonic saline at a rate of 1 mL/kg/h (or 0.5 mL/kg/h in cases with a left ventricular ejection fraction [LVEF] of < 40% or in cases severe congestive heart failure) for at least 2 to 12 hours before and 6 to 24 hours after the procedure. The CRP levels were measured via nephelometry (mg/L) using a Beckman Coulter IMMAGE immunobiochemistry system (Brea, CA).
2.3. Study endpoints
The primary endpoint was CI-AKI, which was defined as an increase in serum creatinine of ≥50% or ≥0.3 mg/dL from baseline within 48 hours.[14] The additional endpoints were CIN25 (contrast-induced nephropathy), which was defined as an increase in serum creatinine of ≥25% or ≥0.5 mg/dL from baseline within 72 hours[15] and CIN0.5, which was defined as an increase in serum creatinine of ≥0.5 mg/dL from baseline within 72 hours.[16] We also recorded the inhospital clinical outcomes, which included acute heart failure, recurrence of acute myocardial infarction, use of an intra-aortic balloon pump (IABP), arrhythmia, stroke, bleeding, and long-term major adverse clinical events (MACE). The MACE outcomes included mortality, nonfatal myocardial infarction, target vessel revascularization, CI-AKI requiring renal replacement therapy, stroke, and rehospitalization.
2.4. Statistical analyses
Normally distributed continuous variables (expressed as mean ± standard deviation) were compared using the t test. Nonnormally distributed continuous variables (expressed as median and interquartile range) were compared using the Wilcoxon rank-sum test. The Pearson χ2 or Fisher exact tests were used, as appropriate, for categorical data (expressed as percentages). Analyses of receiver operating characteristic (ROC) curves were performed to evaluate the ability of CRP levels to predict CI-AKI. The odds ratios (ORs) for CI-AKI in the CRP-tertile subgroups were calculated via unadjusted and adjusted stepwise logistic regression analyses; collinear variables were not retained in the final model. A univariable P = .1 was required to include a variable in the model, and a multivariable P = .05 was required for the variable to remain in the model. Univariable analyses of mortality were performed using the log-rank test, and the multivariable analyses used Cox regression. Our analyses only included cases with available data, and missing data were not imputed. All analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC), and a 2-tailed P < .05 was considered statistically significant.
3. Results
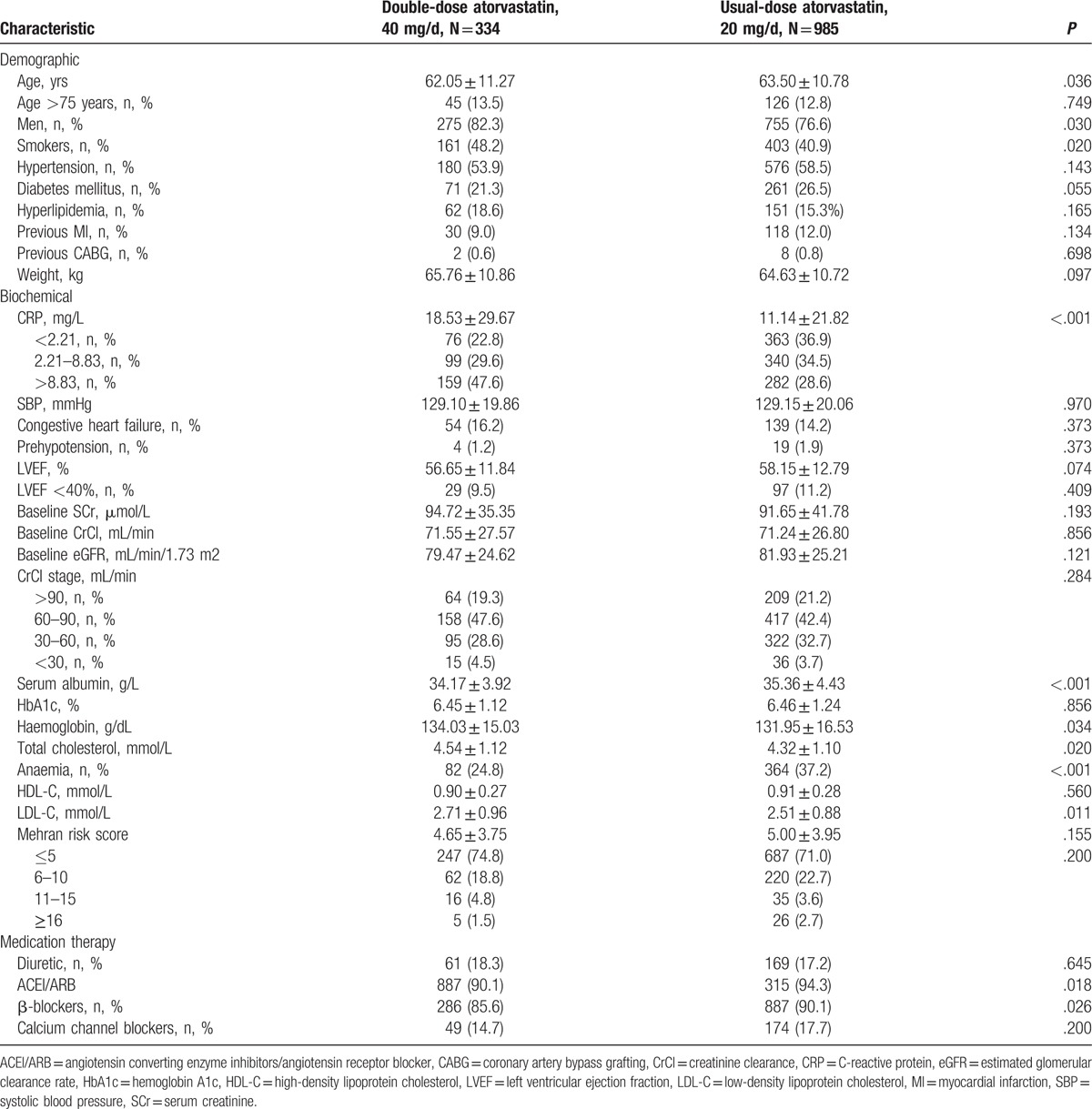
The patients’ baseline characteristics are listed in Table 1. Patients in the double-dose group were generally younger, had higher baseline levels of CRP and LDL-C and had a higher prevalence of anaemia (double- vs usual-dose; baseline CRP: 18.5 ± 29.7 mg/L vs 11.1 ± 21.8 mg/L, P < .001). The use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers was also significantly more frequent in the double-dose group (P = .018). However, there were no significant inter-group differences in their baseline CrCls or mean Mehran scores.
Table 1.
Baseline demographic and clinical characteristics.
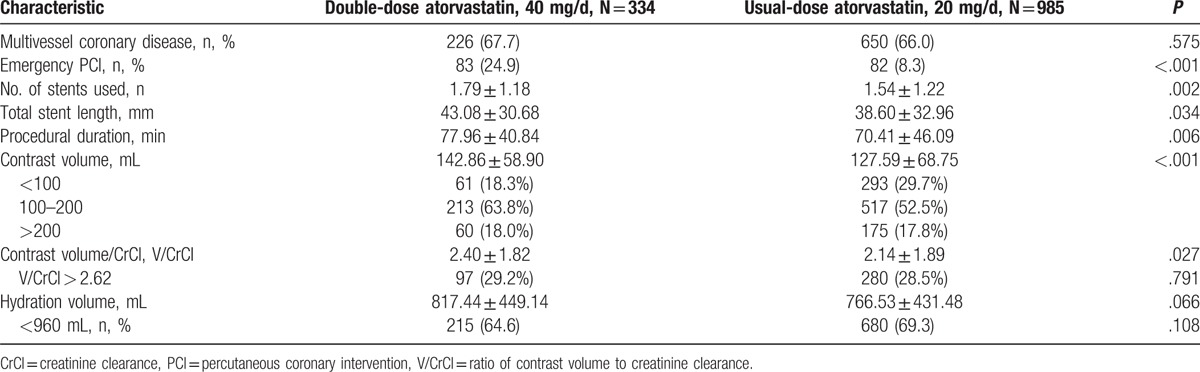
The angiographic and procedural characteristics are listed in Table 2. The double-dose group exhibited a higher frequency of emergency PCI, a greater contrast volume and a longer procedural duration (emergency PCI: 24.9% vs 8.3%, P < .001; contrast volume: 142.9 ± 58.9 mL vs 127.6 ± 68.8 mL, P < .001; procedural duration: 77.96 ± 40.84 minutes vs 70.41 ± 46.09 minutes, P = .006).
Table 2.
Angiographic and procedural characteristics.
3.1. Association of double-dose atorvastatin with CI-AKI and inhospital outcomes
A total of 76 (5.8%) patients developed CI-AKI, including 26 (7.9%) patients in the double-dose group and 50 (5.1%) patients in the usual-dose group (P = .061). This produced a crude OR of 1.59 [95% confidence interval (CI): 0.98–2.61, P = .063). Similar trends were observed in the CRP tertiles (P = .385, .885, and .411 for CRP < 2.21 mg/mL, CRP 2.21–8.83 mg/mL, and CRP > 8.83 mg/mL) and with different definitions (P = .131 and 0.121 for CIN0.5 and CIN25).There were no significant difference in inhospital events such as renal replacement therapy and mortality between the 2 groups (all P > .05). (Tables 3 and 4).
Table 3.
Inhospital clinical outcomes.
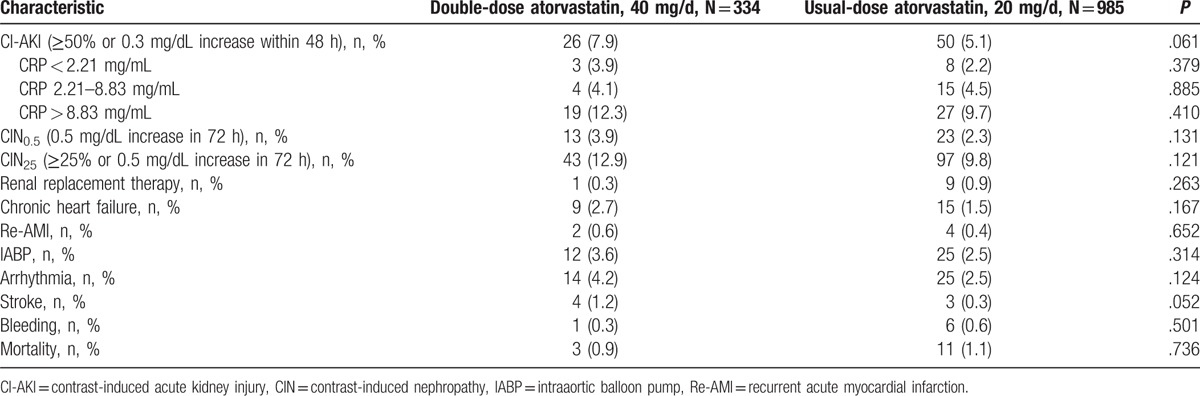
Table 4.
Multivariate analysis of risk factors for contrast-induced acute kidney injury.
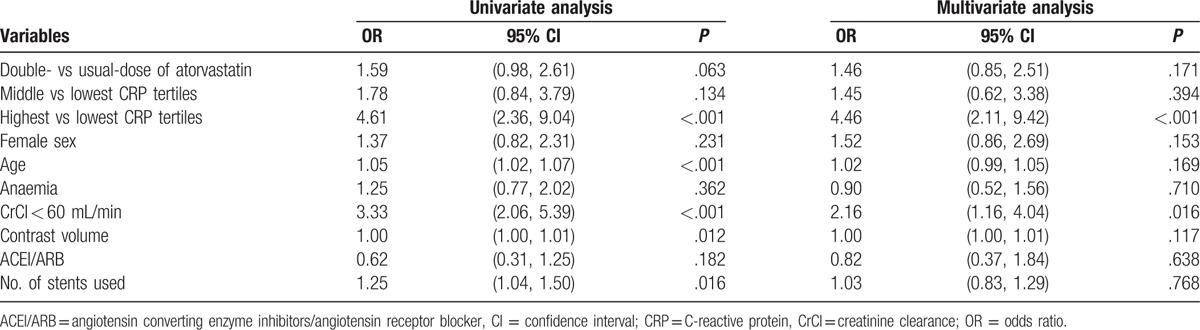
The multivariable logistic regression analysis revealed that double-dose atorvastatin was not associated with a decreased risk of CI-AKI (adjusted OR: 1.46, 95% CI: 0.85–2.51, P = .171), even in patients with the middle CRP levels (adjusted OR: 1.45, 95% CI: 0.62–3.38, P = .394) (Table 4). Similar findings were observed for the other definitions of CIN (CIN25 and CIN0.5). The independent risk factors for CI-AKI were the highest CRP tertile (adjusted OR: 4.46, 95% CI: 2.11–9.42, P < .001), contrast volume and CrCl (Table 4). In the subgroup analysis, double-dose atorvastatin was associated with an increased risk of CI-AKI in patients with a CrCl of <60 mL/min (P = .046), anaemia (P = .009), a contrast volume of >200 mL (P = .024), and >2 stents implanted (P = .026) (Fig. 1).
Figure 1.
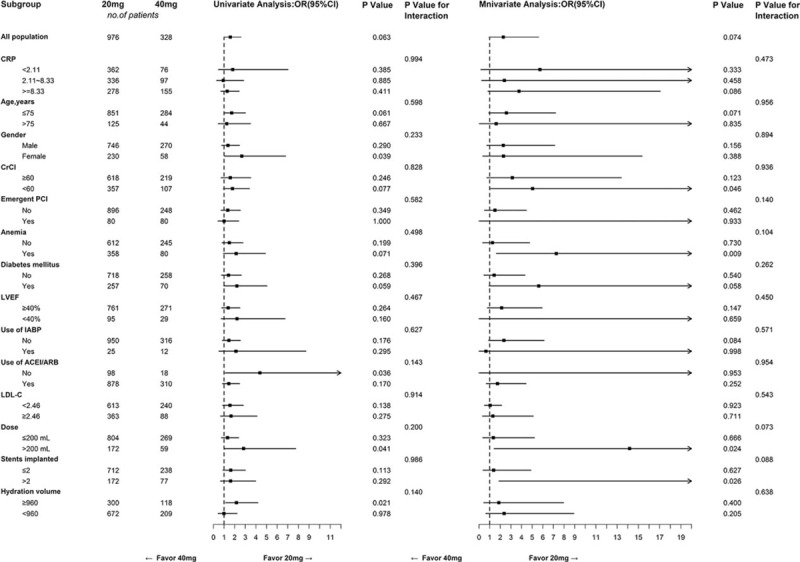
Logistic regression analyses of the double-dose versus usual-dose atorvastatin for predicting contrast-induced acute kidney injury in subgroups. ACEI/ARB = angiotensin converting enzyme inhibitors/angiotensin receptor blockers, CrCl = creatinine clearance, CRP = C-reactive protein, Dose = contrast volume, IABP = intra-aortic balloon pump, LDL-C = low-density lipoprotein cholesterol, LVEF = left ventricular ejection fraction, OR = odds ratio.
3.2. Association of double-dose atorvastatin with long-term outcomes
The median follow-up duration in this cohort was 2.43 years (interquartile range: 1.84–3.24 years). Kaplan-Meier curve analyses revealed that double-dose atorvastatin did not significantly reduce mortality (P = .271) or MACE (P = .383) (Fig. 2). Furthermore, after adjusting for CRP (as a categorical variable) and other confounders, multivariate Cox regression analysis revealed that double-dose atorvastatin was not significantly associated with a reduced risk of mortality [hazard ratio (HR): 0.47, 95% CI: 0.10–2.18] or MACE (HR: 1.03, 95% CI: 0.63–1.69) (Fig. 2). We also did not observe any significant reduction in mortality among patients with (P = .986) or without CI-AKI (P = .888), and in the different CRP tertiles (Fig. 3).Consistent results were observed in multivariate Cox regression for sub-analyses of the highest CRP tertile (Fig. 4).
Figure 2.

Kaplan-Meier curves for the cumulative probability of mortality (A) and MACE (B) in the double-dose and usual-dose groups.
Figure 3.
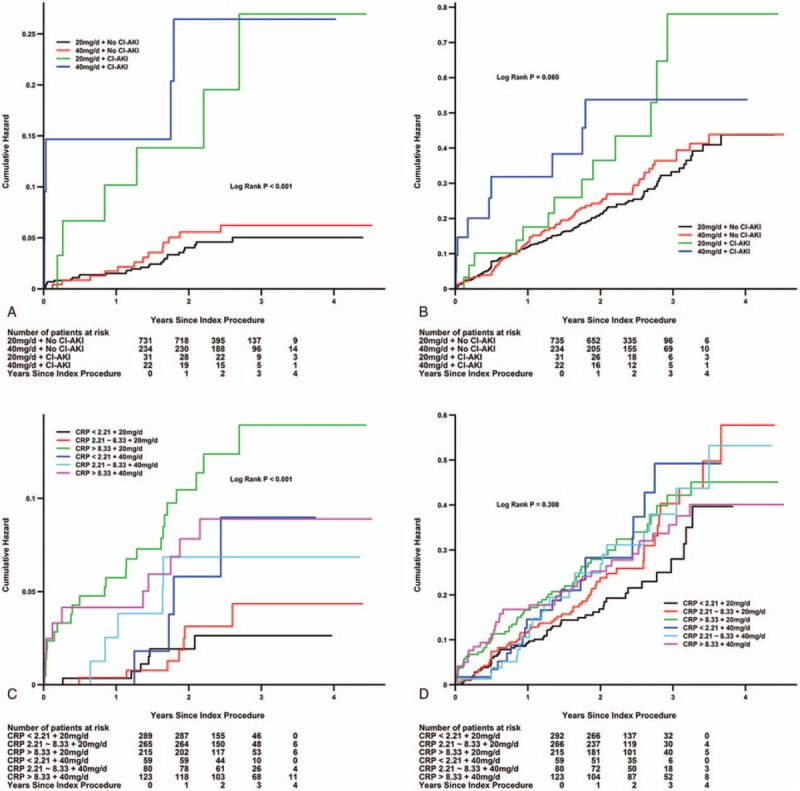
Kaplan-Meier curves for the cumulative probability of mortality (A and C) and MACE (B and D) in the double-dose and usual-dose groups using the model with CI-AKI or CRP tertiles. CRP = C-reactive protein, HR = hazard ratio, MACE = major adverse clinical events.
Figure 4.
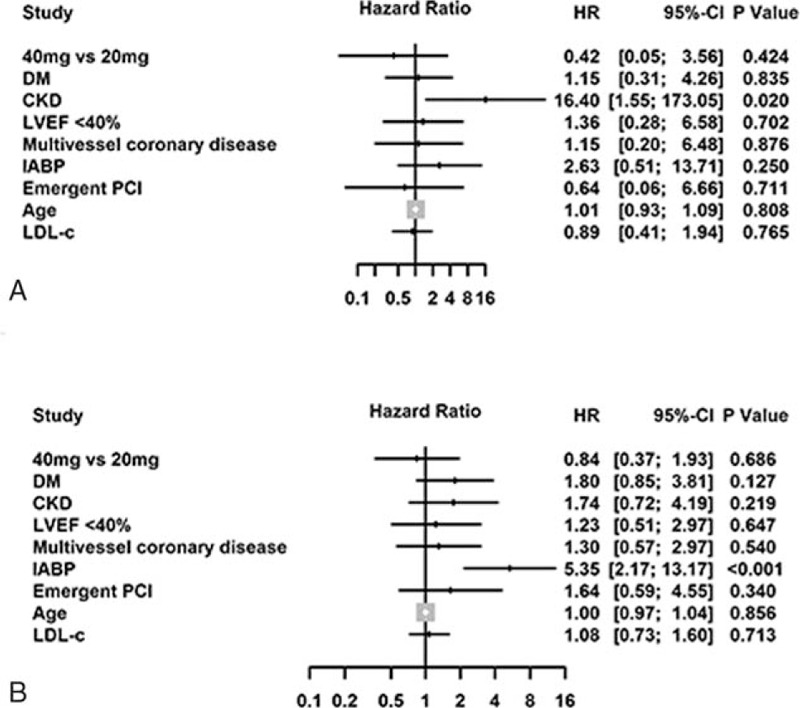
Adjusted risk of mortality (A) and MACE (B) in the highest CRP tertile (>8.33 mg/L) via Cox analysis of double-dose and usual-dose atorvastatin. CKD = chronic kidney disease, defined as creatinine clearance < 60 mL/min, CRP = C-reactive protein, DM = diabetes mellitus, HR = hazard ratio, IABP = intraaortic balloon pump, LDL-C = low-density lipoprotein cholesterol, LVEF = left ventricular ejection fraction, MACE = major adverse clinical events, PCI = percutaneous coronary intervention.
4. Discussion
This study is the first to investigate the associations between common short-term periprocedural doses of atorvastatin (20 or 40 mg/d) and the risk of CI-AKI and patient outcomes after CAG or PCI. Our data suggest that the periprocedural double-dose might be not associated with a reduced risk of CI-AKI and mortality compared with usual-dose, even in patients with high CRP levels, which was an independent risk factor for CI-AKI.
Statin treatment presents an attractive therapy to reduce CI-AKI after CAG or PCI based on preclinical studies of statin mechanisms and studies of CAG. Statins may protect against the effects of contrast medium via decreased endothelin synthesis, the expression of endothelial adhesion molecules and reactive oxygen species production, mechanism implicated in CI-AKI development, and a weakened inflammatory response.[17–20] In this context, CRP is a biomarker for inflammation, which is significantly associated with the risk of CI-AKI, partially because systemic inflammation increases the kidneys’ vulnerability to the local inflammatory processes that are elicited by contrast medium reabsorption.[21–23] Hoshi et al's[24] Propensity score analysis and Morrow et al[25] demonstrated that statin pretreatment was associated with a decrease in the risk of CI-AKI. Moreover, meta-analysis had also confirmed that periprocedural short-term atorvastatin treatment is likely effective in preventing CI-AKI.[26]
Double-dose atorvastatin may increase the risk of CI-AKI, even adjusting for those confounding factors. In a previous study, the patients in the double-dose group had a higher V/CrCl and more frequent emergent PCI, which may have led to the increased risk of CI-AKI.[27,28] Thereby, the occurrence of CI-AKI is associated with incremental cardiovascular events at patients with CKD undergoing emergent PCI.[29] Furthermore, a larger contrast volume was used for the more complicated and longer procedures that were performed among the patients who received double-dose atorvastatin, which also likely increased the risk of CI-AKI.
The common, although minor, difference in the dose of atorvastatin during our treatment without a preprocedural loading dose likely contributed to our finding that double-dose atorvastatin was not more effective in preventing CI-AKI, compared with usual-dose atorvastatin. For example, Patti et al[7] have compared intensive atorvastatin treatment with a loading dose (80 mg/d plus a 40 mg) with a placebo treatment. In that context, a high loading dose of atorvastatin before contrast exposure can reduce the rate of CI-AKI[8,30] and similar results have been reported in studies that used control groups with placebo or no statin treatment.[7,30–33] However, a high daily dose of atorvastatin does not decrease the incidence of CI-AKI (80 vs10 mg/d)[34,35] and in a recent randomized trial, Frederic et al[9] found that high-dose preioperative atorvastatin treatment did not reduce the risk of AKI, which is similar to our findings in the current study (albeit with a smaller difference between the atorvastatin doses). Similarly, no significant differences in clinical benefit have been observed for atorvastatin treatments of 10 mg, 20 mg, and 40 mg.[36] The association between preoperative statin use and postoperative CI-AKI is inconsistent, possibly because of selection bias for statin use, variable effects of treatment, and disparate patient populations. Because Asian ancestry may affect the intensity of statins’ effects, double-dose atorvastatin (40 mg/d) was the most commonly used high-dose treatment at our institution.
Hydration is the gold-standard prophylactic measure for preventing CI-AKI. However, there is no standardized protocol for oral or intravenous periprocedural hydration, and so, we could not evaluate the preventative effect of oral hydration. Nevertheless, oral hydration may be as effective as intravenous rehydration for preventing CI-AKI.[37,38] Thus, it is possible that the different hydration volumes between the 2 dose groups might have influenced the observed benefit of double-dose atorvastatin.
4.1. Limitations of the study
The current study has several limitations. First, this prospective observational study did not use randomization, and was conducted at a single centre. Second, the CrCl was calculated using the Cockcroft-Gault formula, rather than via direct measurement. Third, variations in the measurement times may have caused us to miss postprocedure peak creatinine levels. Furthermore, approximately half of the patients were discharged at 2 days after CAG, and so, their serum creatinine concentrations could not be measured on day 2. Thus, this variation and lack of data may have led to an underestimation of the true CI-AKI incidence in the study population. Fourth, we were unable to obtain CRP data during the follow up. Fifth, the time from statin loading to contrast exposure varied in different patients. Lastly, there is no standardized protocol for oral or intravenous periprocedural hydration.
5. Conclusions
Our observational data suggest that daily periprocedural double-dose atorvastatin (the commonly used dose in clinical practice) was not associated with a reduced risk of CI-AKI, even in patients with high CRP levels, which was an independent risk factor for CI-AKI. However, daily periprocedural double-dose atorvastatin was associated with an increased risk of CI-AKI in patients with risk factors such as a CrCl of <60 mL/min and a contrast volume of >200 mL. In addition, double-dose atorvastatin did not improve the long-term prognosis. Nevertheless, well-designed and adequately-powered randomized controlled trials are needed to confirm these conclusions.
Footnotes
Abbreviations: CAG = coronary angiography, CI = confidence interval, CI-AKI = contrast-induced acute kidney injury, CIN = contrast-induced nephropathy, CrCl = creatinine clearance, CRP = C-reactive protein, HR = hazard ratio, IABP = intra-aortic balloon pump, LVEF = left ventricular ejection fraction, MACE = major adverse clinical events, OR = odds ratio, PCI = percutaneous coronary intervention, ROC = receiver operating characteristic.
Wei-Jie Bei and Shi-Qun Chen contributed equally to this work.
The study was supported by the grants from the National Natural Science Foundation of China (grant no.: 81270286), Guangdong Cardiovascular Institute and the Guangdong Cardiovascular Institute's Guangdong Provincial Cardiovascular Clinical Medicine Research Fund (grant no.: 2009X41), the Science and Technology Planning Project of Guangdong Province (grant no.: 2012A030400039), and the Science and Technology Project of Guangzhou City (grant no.: 2014Y2-00191). The funders had no role in the study design, data collection, and analysis; decision to publish; or preparation of the article. The study was not funded by any industry.
The authors declare no conflicts of interest.
References
- [1].Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 2002;105:2259–64. [DOI] [PubMed] [Google Scholar]
- [2].Gupta R, Gurm HS, Bhatt DL, et al. Renal failure after percutaneous coronary intervention is associated with high mortality. Catheter Cardiovasc Interv 2005;64:442–8. [DOI] [PubMed] [Google Scholar]
- [3].McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol 2008;51:1419–28. [DOI] [PubMed] [Google Scholar]
- [4].Tumlin J, Stacul F, Adam A, et al. Pathophysiology of contrast-induced nephropathy. Am J Cardiol 2006;98:14K–20K. [DOI] [PubMed] [Google Scholar]
- [5].Kuller LH, Tracy RP, Shaten J, et al. Relation of C-reactive protein and coronary artery disease in the MRFIT nested case-control study. Multiple risk factor intervention trial. Am J Epidemiol 1996;144:537–47. [DOI] [PubMed] [Google Scholar]
- [6].Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–207. [DOI] [PubMed] [Google Scholar]
- [7].Patti G, Ricottini E, Nusca A, et al. Short-term, high-dose atorvastatin pretreatment to prevent contrast-induced nephropathy in patients with acute coronary syndromes undergoing percutaneous coronary intervention (from the ARMYDA-CIN [atorvastatin for reduction of myocardial damage during angioplasty--contrast-induced nephropathy] trial. Am J Cardiol 2011;108:1–7. [DOI] [PubMed] [Google Scholar]
- [8].Zhou X, Jin YZ, Wang Q, et al. Efficacy of high dose atorvastatin on preventing contrast induced nephropathy in patients underwent coronary angiographys. Zhonghua Xin Xue Guan Bing Za Zhi 2009;37:394–6. [PubMed] [Google Scholar]
- [9].Billings FT, 4th, Hendricks PA, Schildcrout JS, et al. High-dose perioperative atorvastatin and acute kidney injury following cardiac surgery: a randomized clinical trial. JAMA 2016;315:877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tan N, Liu Y, Zhou YL, et al. Contrast medium volume to creatinine clearance ratio: a predictor of contrast-induced nephropathy in the first 72 hours following percutaneous coronary intervention. Catheter Cardiovasc Interv 2012;79:70–5. [DOI] [PubMed] [Google Scholar]
- [11].Stacul F, van der Molen AJ, Reimer P, et al. Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR). Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol 2011;21:2527–41. [DOI] [PubMed] [Google Scholar]
- [12].Wright RS, Anderson JL, Adams CD, et al. 2011 ACCF/AHA focused update of the Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction (updating the 2007 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011;57:1920–59. [DOI] [PubMed] [Google Scholar]
- [13].Xun L, Cheng W, Hua T, et al. Assessing glomerular filtration rate (GFR) in elderly Chinese patients with chronic kidney disease (CKD): a comparison of various predictive equations. Arch Gerontol Geriatr 2010;51:13–20. [DOI] [PubMed] [Google Scholar]
- [14].Moriyama N, Ishihara M, Noguchi T, et al. Admission hyperglycemia is an independent predictor of acute kidney injury in patients with acute myocardial infarction. Circ J 2014;78:1475–80. [DOI] [PubMed] [Google Scholar]
- [15].Ivanes F, Isorni MA, Halimi JM, et al. Predictive factors of contrast-induced nephropathy in patients undergoing primary coronary angioplasty. Arch Cardiovasc Dis 2014;107:424–32. [DOI] [PubMed] [Google Scholar]
- [16].Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med 2006;354:379–86. [DOI] [PubMed] [Google Scholar]
- [17].Hernández-Perera O, Pérez-Sala D, Navarro-Antolín J, et al. Effects of the 3-hy-droxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest 1998;101:2711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Patti G, Chello M, Pasceri V, et al. Protection from procedural myocardial injury by atorvastatin is associated with lower levels of adhesion molecules after percutaneous coronary intervention: results from the ARMYDA-CAMs (atorvastatin for reduction of myocar-dial damage during angioplasty-cell adhesion molecules) substudy. J Am Coll Cardiol 2006;48:1560–6. [DOI] [PubMed] [Google Scholar]
- [19].Rikitake Y, Kawashima S, Takeshita S, et al. Anti-oxidative proper-ties of fluvastatin, an HMG-CoA reductase inhibitor, contribute to prevention of atherosclerosis in cholesterol-fed rabbits. Atherosclerosis 2001;154:87–96. [DOI] [PubMed] [Google Scholar]
- [20].Bonetti PO, Lerman LO, Napoli C, et al. Statin effects beyond lipid lowering-are they clinically relevant? Eur Heart J 2003;24:225–48. [DOI] [PubMed] [Google Scholar]
- [21].Toso A, Leoncini M, Maioli M, et al. Relationship between inflammation and benefits of early high-dose rosuvastatin on contrast-induced nephropathy in patients with acute coronary syndrome: the pathophysiological link in the PRATO-ACS study (Protective effect of rosuvastatin and antiplatelet therapy on contrast-induced nephropathy and myocardial damage in patients with acute coronary syndrome undergoing coronary intervention). JACC Cardiovasc Interv 2014;7:1421–9. [DOI] [PubMed] [Google Scholar]
- [22].Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003;107:363–9. [DOI] [PubMed] [Google Scholar]
- [23].Kwasa EA, Vinayak S, Armstrong R. The role of inflammation in contrast-induced nephropathy. Br J Radiol 2014;87:20130738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hoshi T, Sato A, Kakefuda Y, et al. Preventive effect of statin pretreatment on contrast-induced acute kidney injury in patients undergoing coronary angioplasty: propensity score analysis from a multicenter registry. Int J Cardiol 2014;171:243–9. [DOI] [PubMed] [Google Scholar]
- [25].Morrow DA, de Lemos JA, Sabatine MS, et al. Clinical relevance of C-reactive protein during follow-up of patients with acute coronary syndromes in the Aggrastat-to-Zocor Trial. Circulation 2006;114:281–8. [DOI] [PubMed] [Google Scholar]
- [26].Takagi H, Umemoto T. A meta-analysis of randomized trials for effects of periprocedural atorvastatin on contrast-induced nephropathy. Int J Cardiol 2011;153:323–5. [DOI] [PubMed] [Google Scholar]
- [27].Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 2004;44:1393–1393. [DOI] [PubMed] [Google Scholar]
- [28].Fu N, Li X, Yang S, et al. Risk score for the prediction of contrast-induced nephropathy in elderly patients undergoing percutaneous coronary intervention. Angiology 2013;64:188–94. [DOI] [PubMed] [Google Scholar]
- [29].Watabe H, Sato A, Hoshi T, et al. Association of contrast-induced acute kidney injury with long-term cardiovascular events in acute coronary syndrome patients with chronic kidney disease undergoing emergent percutaneous coronary intervention. Int J Cardiol 2014;174:57–63. [DOI] [PubMed] [Google Scholar]
- [30].Quintavalle C, Fiore D, De Micco F, et al. Impact of a high loading dose of atorvastatin on contrast-induced acute kidney injury. Circulation 2012;126:3008–16. [DOI] [PubMed] [Google Scholar]
- [31].Li W, Fu X, Wang Y, et al. Beneficial effects of high-dose atorvastatin pretreatment on renal function in patients with acute ST-segment elevation myocardial infarction undergoing emergency percutaneous coronary intervention. Cardiology 2012;122:195–202. [DOI] [PubMed] [Google Scholar]
- [32].Ozhan H, Erden I, Ordu S, et al. Efficacy of short-term high-dose atorvastatin for prevention of contrast-induced nephropathy in patients undergoing coronary angiography. Angiology 2010;61:711–4. [DOI] [PubMed] [Google Scholar]
- [33].Bach RG. ACP Journal Club: review: periprocedural high-dose statins reduce contrast-induced acute kidney injury after coronary angiography. Ann Intern Med 2015;162:JC4. [DOI] [PubMed] [Google Scholar]
- [34].Toso A, Maioli M, Leoncini M, et al. Usefulness of atorvastatin (80 mg) in prevention of contrast-induced nephropathy in patients with chronic renal disease. Am J Cardiol 2010;105:288–92. [DOI] [PubMed] [Google Scholar]
- [35].Jo SH, Hahn JY, Lee SY, et al. High-dose atorvastatin for preventing contrast-induced nephropathy in primary percutaneous coronary intervention. J Cardiovasc Med (Hagerstown) 2015;16:213–9. [DOI] [PubMed] [Google Scholar]
- [36].Zhao SP, Yu BL, Peng DQ, et al. The effect of moderate-dose versus double-dose statins on patients with acute coronary syndrome in China: Results of the CHILLAS trial. Atherosclerosis 2014;233:707–12. [DOI] [PubMed] [Google Scholar]
- [37].Kong DG, Hou YF, Ma LL, et al. Comparison of oral and intravenous hydration strategies for the prevention of contrast-induced nephropathy in patients undergoing coronary angiography or angioplasty: a randomized clinical trial. Acta Cardiol 2012;67:565–9. [DOI] [PubMed] [Google Scholar]
- [38].Hiremath S, Akbari A, Shabana W, et al. Prevention of contrast-induced acute kidney injury: is simple oral hydration similar to intravenous? A systematic review of the evidence. PLoS One 2013;8:e60009. [DOI] [PMC free article] [PubMed] [Google Scholar]


