Abstract
Liver resection may be beneficial in intermediate-stage hepatocellular carcinoma (HCC), though the benefit of postoperative anti-hepatitis B virus (HBV) therapy in these patients remains unclear. In this study, we sought to evaluate the efficacy of postoperative anti-HBV for intermediate-stage HCC patients who underwent radical liver resection.
According to inclusion and exclusion criteria, this study enrolled 202 HCC patients who underwent liver resection and had a high HBV-DNA load. The patients were divided into 2 groups on the basis of postoperative anti-HBV therapy: group A included patients undergoing postoperative anti-HBV therapy, whereas group B patients did not receive any postoperative anti-HBV therapy. Factors including baseline demographics, tumor characteristics, overall long-term survival, tumor-free survival, and tumor recurrence rate were compared between the 2 groups. Moreover, univariate and multivariate analyses were used to identify risk factors of HCC recurrence.
Baseline demographics and tumor characteristics were comparable between the groups. The 1-, 3-, and 5-year overall survival rates in group A were 91.3%, 80.9%, and 66.1%, respectively, values that were significantly increased compared with group B (91.7%, 60.7%, and 52.4%, respectively, P = .019). Group A patients also exhibited enhanced 1-, 3-, and 5-year tumor-free survival compared with group B patients (87.0%, 67.0%, and 62.6%, respectively, in group A; 82.1%, 50.0%, and 42.9% in group B, P = .002). In addition, the tumor recurrence rate in group B was significantly increased compared with group A (P < .01). Univariate and multivariate analyses indicated lack of postoperative anti-HBV therapy [hazard ratio (HR) = 0.882; 95% confidence interval (CI), 0.712–0.938; P = .042] to be a predictor of tumor recurrence.
For intermediate-stage [Barcelona Clinic Liver Cancer (BCLC) stage B] HCC with a high HBV-DNA load, postoperative anti-HBV therapy after curative resection should be routine adjuvant therapy.
Keywords: hepatitis B virus, hepatocellular carcinoma, liver, recurrence
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies[1] and the leading cause of cancer-related death worldwide.[2] Chronic infections with hepatitis B virus (HBV) and hepatitis C virus (HCV) account for approximately 75% to 80% of HCCs.[3] In particular, HBV infection is a predominant risk factor for HCC in Asia and Africa.[4,5] Currently, hepatic resection, liver transplantation (LT), and radiofrequency ablation (RFA) are the curative treatments for early-stage HCC.[4] However, the high recurrence rate remains a major obstacle for improving outcomes. The mechanisms of HCC recurrence primarily involve intrahepatic metastasis or multicentric carcinogenesis within a background of viral liver disease.[6] Various factors, including a high load of HBV-DNA, are considered a risk for HCC recurrence after radical therapy.[7] In recent years, several studies have indicated that a high HBV-DNA load predicts HCC recurrence after curative resection.[8,9] However, few studies have evaluated the effectiveness of postoperative anti-HBV therapy on tumor recurrence.
LT should be the first line of treatment for early-stage HCC patients; however, for intermediate-stage HCC patients [Barcelona Clinic Liver Cancer (BCLC) stage B], transcatheter hepatic arterial chemoembolization (TACE) is the first choice in Europe[10] and America.[11] In contrast, according to Chinese guidelines,[12] liver resection is the first choice due to the potential benefit compared with TACE.[13–15]
To our knowledge, no reports published to date have demonstrated the effectiveness of postoperative anti-HBV therapy in intermediate-stage HCC patients. Therefore, we herein evaluate the significance of postoperative anti-HBV therapy in intermediate-stage HCC patients with a high HBV-DNA load.
2. Patients and methods
2.1. Patients
This study included 202 intermediate-stage HCC patients with a high HBV-DNA load who underwent liver resection as initial radical therapy at our center between January 2005 and January 2009. This study was approved by the ethics committee and institutional review board of The First Hospital, Lanzhou University. Informed consent was signed by the patients or their families. We divided these patients into 2 groups on the basis of the presence or absence of postoperative anti-hepatitis B virus therapy: group A received postoperative anti-HBV therapy with lamivudine (LDV); group B did not receive postoperative anti-HBV therapy due to patient unwillingness. Diagnosis of HCC was based on American Association for the Study of Liver Disease (AASLD) guidelines.[16] Intermediate-stage HCCs were graded using the BCLC staging system[17]; BCLC stage B was defined as having 1 lesion >5 cm in diameter or 2 to 3 lesions (of which at least 1 was >3 cm in diameter) or more than 3 lesions of any diameter.[13,14] The following inclusion criteria were used for this study: age of 18 to 70 years; positive serum HBV-DNA (>3 log10 copies/mL) at the time of resection; intermediate-stage HCC (BCLC B); Child Score A; liver resection performed as the initial therapy; and HCC proven by postoperative histopathological examination. The exclusion criteria for this study were as follows: the presence of preoperative therapies; HCV or alcohol liver cirrhosis; absence of follow-up; and absence of HBV-DNA.
2.2. Liver resection procedure
All surgical procedures were performed by 1 of the 5 surgeons at the institute with at least 20 years of experience in hepatobiliary surgery and while the patient was under general anesthesia. Anatomical resection was based on the segmental division of the liver; however, nonanatomical resection with a sufficient resection margin was often adopted to ensure that an adequate volume of liver was conserved. HCC resection was performed anatomically with at least 1 to 3 cm of nontumor margin.
2.3. Follow-up
After resection, the patients received follow-up and were monitored regularly. Ultrasonography was the first choice of follow-up imaging. When recurrence was suspected, dynamic contrast-enhanced computer tomography (CT) or magnetic resonance imaging (MRI) was selected to confirm this event. Elevated or persistent increases in alpha-fetoprotein (AFP) levels may also indicate HCC recurrence. Therapies administered after HCC recurrence largely depended on the patient's liver function and tumor characteristics and included re-resection, RFA, TACE, high-intensity focused ultrasound (HIFU), and LT. No HCC patient took Sorafenib as adjuvant therapy. In addition, no other chemotherapy or radiotherapy was recommended according to the guidelines.
2.4. Statistical analysis
Data were compared by analysis of variance for continuous variables and by Chi-square or Mantel-trend tests for categorical variables. Differences between the 2 groups were analyzed using the log-rank test if necessary. Overall and tumor-free survival rates were calculated using Kaplan–Meier analysis; the significance of a difference between the 2 groups was assessed using the log-rank test. Variables with a P value less than .05 in univariate analysis were included in multivariate analysis. All P values were 2-tailed, and P values <.05 were considered significant. Statistical analyses were performed using SPSS (SPSS 15.0 Inc., Chicago, IL).
3. Results
3.1. Baseline characteristics
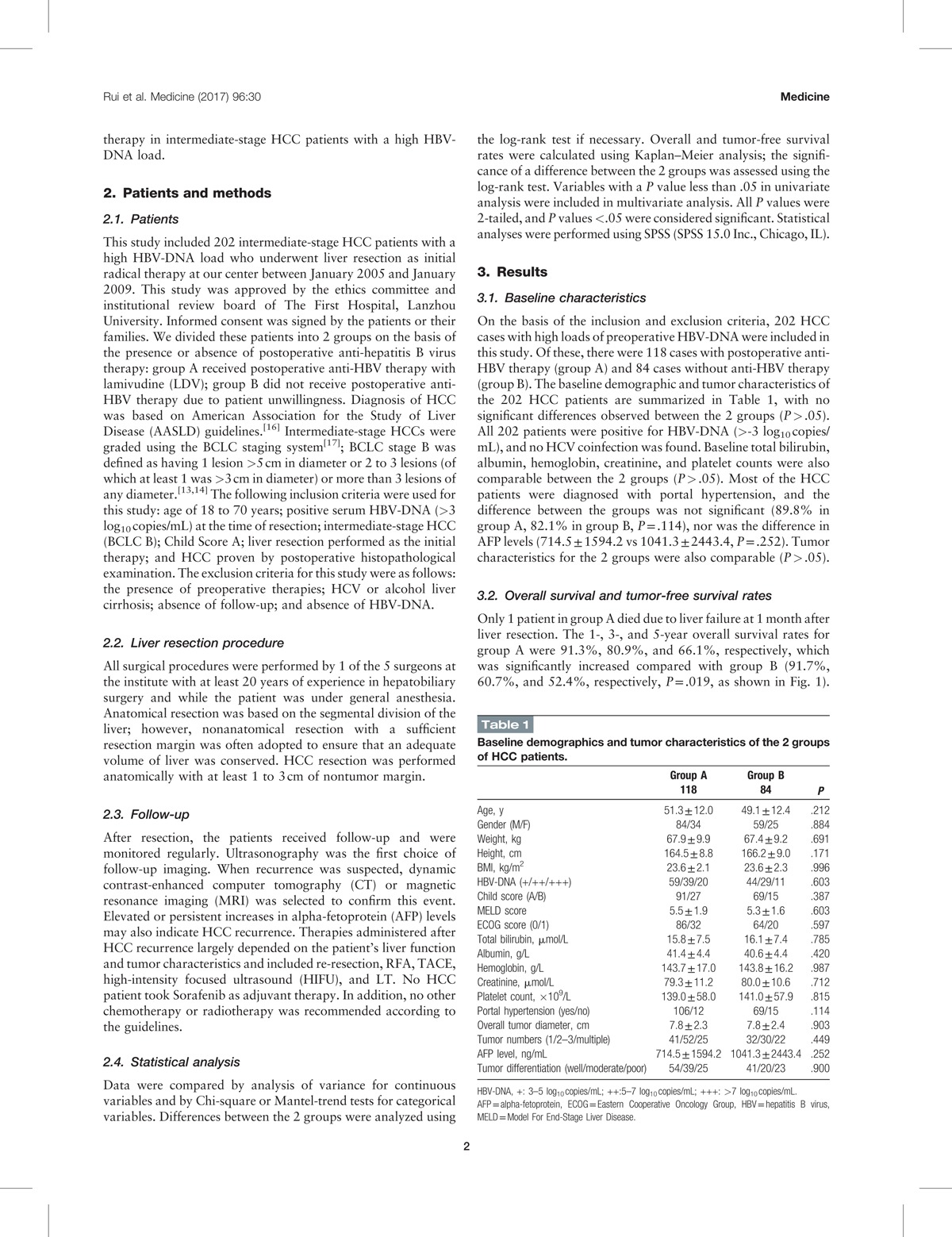
On the basis of the inclusion and exclusion criteria, 202 HCC cases with high loads of preoperative HBV-DNA were included in this study. Of these, there were 118 cases with postoperative anti-HBV therapy (group A) and 84 cases without anti-HBV therapy (group B). The baseline demographic and tumor characteristics of the 202 HCC patients are summarized in Table 1, with no significant differences observed between the 2 groups (P > .05). All 202 patients were positive for HBV-DNA (>-3 log10 copies/mL), and no HCV coinfection was found. Baseline total bilirubin, albumin, hemoglobin, creatinine, and platelet counts were also comparable between the 2 groups (P > .05). Most of the HCC patients were diagnosed with portal hypertension, and the difference between the groups was not significant (89.8% in group A, 82.1% in group B, P = .114), nor was the difference in AFP levels (714.5 ± 1594.2 vs 1041.3 ± 2443.4, P = .252). Tumor characteristics for the 2 groups were also comparable (P > .05).
Table 1.
Baseline demographics and tumor characteristics of the 2 groups of HCC patients.
3.2. Overall survival and tumor-free survival rates
Only 1 patient in group A died due to liver failure at 1 month after liver resection. The 1-, 3-, and 5-year overall survival rates for group A were 91.3%, 80.9%, and 66.1%, respectively, which was significantly increased compared with group B (91.7%, 60.7%, and 52.4%, respectively, P = .019, as shown in Fig. 1). Group A patients also exhibited enhanced 1-, 3-, and 5-year tumor-free survival compared with group B patients (87.0%, 67.0%, and 62.6%, respectively, in group A; 82.1%, 50.0%, and 42.9%, respectively, in group B, P = .002; Fig. 2).
Figure 1.
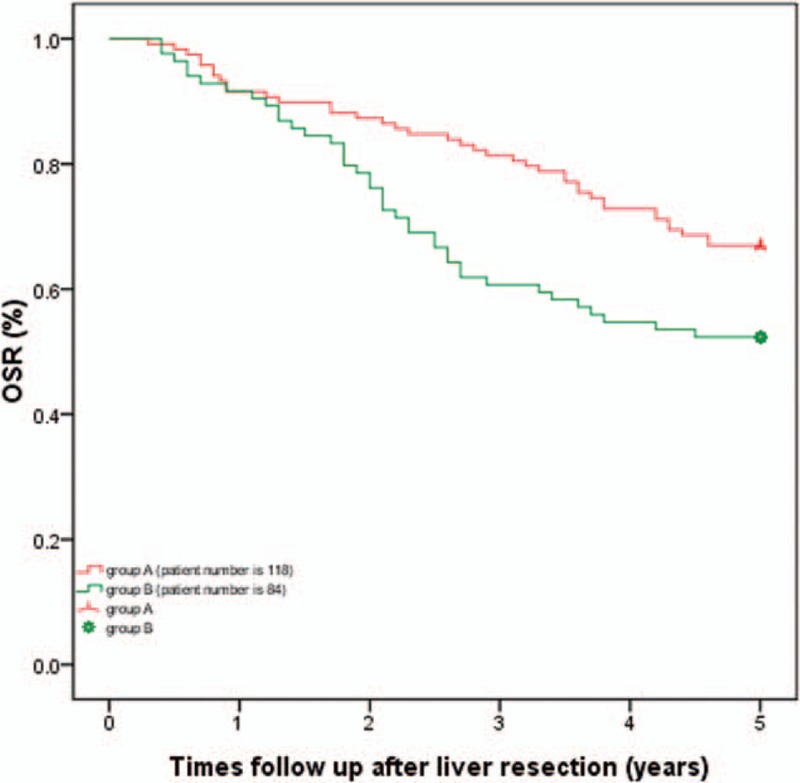
Comparison of 1-, 3-, and 5-year overall survival rates: The 1-, 3-, and 5-year overall survival rates for group A were 91.3%, 80.9%, and 66.1%, respectively, which was significantly increased compared with group B (91.7%, 60.7%, and 52.4%, respectively, P = .019).
Figure 2.
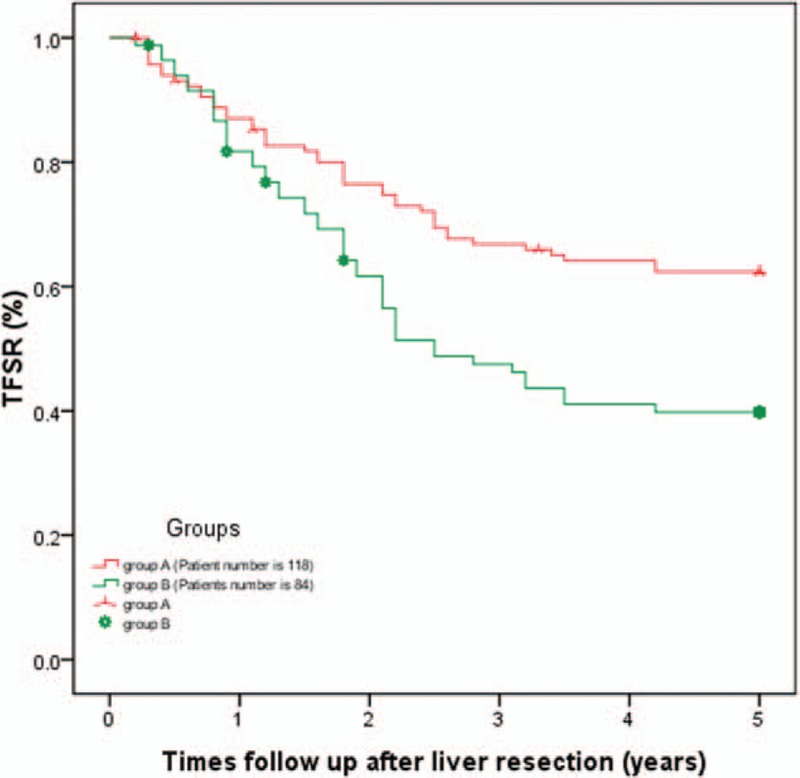
Comparison of 1-, 3-, and 5-year tumor-free survival between the 2 groups: Group A patients exhibited enhanced 1-, 3-, and 5-year tumor-free survival compared with group B patients (87.0%, 67.0%, and 62.6%, respectively, in group A; 82.1%, 50.0%, and 42.9%, respectively, in group B, P = .002).
3.3. Univariate and multivariate analyses
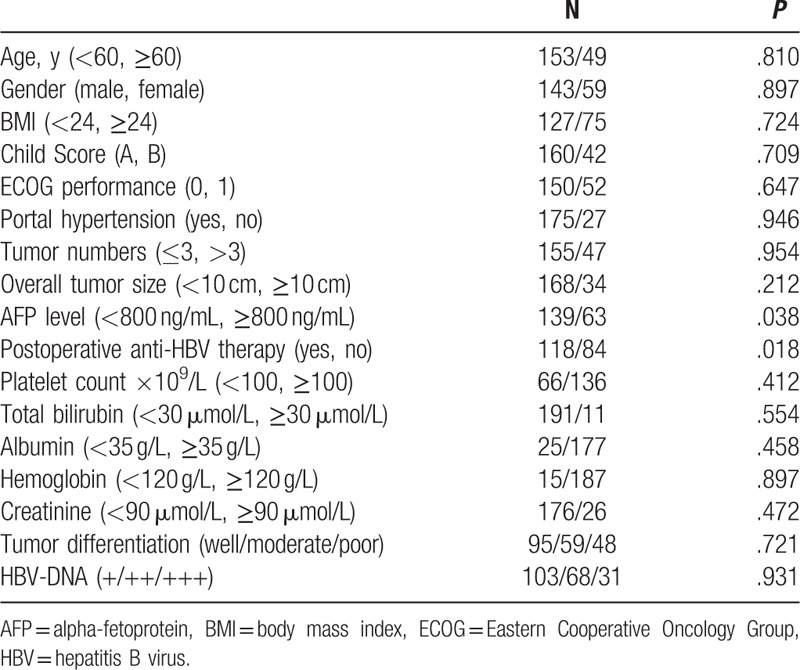
Univariate survival analysis of tumor recurrence was performed using 18 variables (Table 2): age, gender, body mass index (BMI), HBV-DNA level, Child Score, Eastern Cooperative Oncology Group (ECOG) performance, portal hypertension, tumor number, overall tumor size, AFP level, platelet count ×109/L, total bilirubin, albumin, hemoglobin, creatinine, tumor differentiation, and postoperative anti-HBV therapy. According to univariate analysis, the absence of postoperative anti-HBV therapy and a high AFP level (≥800 ng/mL) were predictors of tumor recurrence after liver resection among the total population. Multivariate analysis confirmed that the absence of postoperative anti-HBV therapy [hazard ratio (HR) = 0.882; 95% confidence interval (CI), 0.712–0.938; P = .042] was a predictor of tumor recurrence for BCLC-B HCC patients who underwent liver resection.
Table 2.
Univariate analyses of tumor recurrence in intermediate HCC patients.
3.4. Tumor recurrence and treatment
Among the 202 patients who underwent surgical resection, recurrence or metastasis of HCC occurred in 90 patients during the follow-up period (68 cases with liver recurrence and 22 cases with other organ metastasis). The remnant liver (86 cases, 95.6%) was the most common site of recurrence or metastasis, and the lungs and abdominal lymph nodes were the most common organs or tissue sites for extrahepatic metastasis, followed by bone. Treatment of the recurrence or metastasis of HCC mainly depended on liver function, recurrence site, tumor location, and recurrent tumor characteristics, primarily involving re-resection, TACE, RFA, HIFU. In the 5 years of follow-up, 79 patients died: 50 due to HCC recurrence, 16 due to decompensated liver cirrhosis, 12 due to both of these reasons, and 1 due to cardiac failure.
4. Discussion
HCC is one of the most common human cancers and a leading cause of cancer-related death worldwide. Chronic HBV and HCV infection and alcohol abuse are the most prominent risk factors for HCC. However, in Asian countries, especially in China, HBV infection is the main cause for HCC. Universal HBV vaccination of newborn babies has substantially reduced the incidence of HCC in the recent 3 decades,[9] yet adult patients with HBV infection have a major risk of liver failure or HCC occurrence, and the death rate during their lifetime could reach approximately 40%.[18] The most effective methods for reducing the risk of liver failure or HCC include the use of antiviral agents. Although LT, liver resection, and RFA are among potential radical therapies for early-stage HCC, the low survival rate of HBV-related HCC is attributed to its high recurrence rate. Indeed, despite a 50% to 70% 5-year overall survival rate, HCC recurs in up to 80% of the patients.[19] To date, most of the studies focusing on the benefit of postoperative anti-HBV virus therapy[20] involved early-stage HCC (BCLC stage A) because liver resection is only recommended for early-stage HCC in Europe[10] and America.[11] Nonetheless, it has recently been argued that intermediate-stage HCC can also benefit from liver resection when comparing with TACE.[15,21] However, the correlation between anti- HBV therapy and the benefit to intermediate-stage HCC after curative resection is still unclear. Thus, we sought to evaluate the benefits of postoperative anti-HBV therapy in BCLC-B HCC patients who accepted liver resection and for whom long-term follow-up was performed.
The potential mechanism of hepatocarcinogenesis induced by hepatitis B virus includes direct transformation and some indirect effects.[22] Persistent infection by hepatitis B virus may result in hepatocyte destruction and then regeneration may increase genetic instability, and this is an indirect effect.[23] Progression of liver damage is associated with persistent viral replication and may lead to liver fibrosis and cirrhosis. The goal of anti-HBV therapy is to reduce the viral load and liver inflammation.[24] In addition, antivirus therapy potentially improves liver function in decompensated patients.[25] As demonstrated in several studies, high HBV-DNA serum levels are a predictor for HCC development,[26–28] and antivirus therapy effectively inhibits HBV replication.[29] Patients administered anti-HBV therapy exhibit a reduced rate of HCC compared with those with virus breakthrough or untreated controls.[30] Although curative resection provides a complete cure for HCC, recurrence is a major concern and typically occurs within 2 years of surgery. Therefore, whether antivirus therapy after hepatic resection prevents HCC recurrence is an important issue. Chan et al[31] reported that overall and tumor-free survival rates were increased in patients receiving antivirus treatment after hepatectomy compared with those who did not receive antivirus therapy. The 1-, 3-, and 5-year overall survival rates in the treatment group were 88.1%, 79.1%, and 71.2%, respectively, slightly higher than our treatment group. The main reason for these differences may be that our study included HCC patients at BCLC stage B, whereas the effect of anti-HBV therapy on reducing HCC recurrence is less apparent in patients at BCLC stage C.[32] Thus, predictive overall survival was too short to observe the effect of the antivirus therapy. Accordingly, the effect of anti-HBV therapy on BCLC stage B HCC remains unclear. Univariate and multivariate analyses also demonstrated that the absence of postoperative anti-HBV therapy may be a risk factor for tumor recurrence, even after curative liver resection. Overall, long-term antivirus therapy in adults reduces but does not prevent HCC recurrence after radical therapy. Moreover, this therapy can cause complications, including decompensated cirrhosis.[33]
The current approved medications for HBV treatment include interferon-α (IFNα) and nucleotide analogs (NAs), including LVD, entecavir (ETV), tenofovir disoproxil fumarate, adefovir-dipivoxil (ADV), and telbivudine.[34] Various potential clinical problems are associated with the use of IFN, including the method of administration, potential severe side effects for certain subgroups of Asian patients, and limited applicability.[9] In this study, none of the HCC patients who underwent curative treatment received IFN therapy. Three nucleoside analogs (LDV, ETV, and telbivudine) and 2 NAs (adefovir and tenofovir) are currently Food and Drug Administration (FDA)-approved for the treatment of hepatitis B. These analogs selectively target HBV-NDA.[35] As ETV is a potent antiviral agent with a high genetic barrier to resistance, it is currently recommended as a first-line antiviral therapy for HBV. Regardless, our study included all patients from January 2005 to January 2009, and LDV with or without adefovir was the first-line anti-HBV therapy at this time in mainland China.[36] A noticeable rate of occurrence and recurrence of HCC is observed during and after therapeutic courses, as shown by our results. The main reason for this rate may be that antiviral reagents cannot eliminate viruses in hepatocytes that exist in the form of covalently closed circular DNA.[37] In addition, the viruses may have already integrated into the host genome before the onset of therapy, resulting in genomic alteration and chromosomal instability.[38]
Some limitations of the present study should be noted. First, this is a retrospective study from a single center. Second, this is a single-center analysis from China, which has a high prevalence of HBV infection; therefore, it is possible that our results may be not applicable to other cases of HBV-related HCC. Third, all HCC cases were intermediate stage (BCLCB), and very early, early, or advanced HCCs were not included. Lastly, patients undergoing other radical therapies, such as RFA or LT, were excluded. Therefore, larger, more comprehensive, randomized multicenter studies are needed to confirm our results.
In conclusion, intermediate-stage HCC patients with a high HBV-DNA load may benefit from postoperative anti-HBV therapy after radical hepatic resection. Accordingly, postoperative anti-HBV therapy should be a routine adjuvant therapy for BCLC stage B HCC.
Acknowledgments
We thank LJ from Sichuan University for assistance with the study design and the English editing of our manuscript. We are also grateful for the grants from the Youth Science and Technology Fund (1506JYA262).
Footnotes
Abbreviations: AASLD = American Association for the Study of Liver Disease, AFP = alpha-fetoprotein, BCLC = Barcelona Clinic Liver Cancer, BMI = body mass index, CUSA = cavitron ultrasonic surgical aspirator, ECOG = Eastern Cooperative Oncology Group, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, HIFU = high-intensity focused ultrasound, LDV = lamivudine, LT = liver transplantation, MELD = Model for End-Stage Liver Disease, RFA = radiofrequency ablation.
Authorship: All authors contributed to the design and interpretation of the study and to further drafts. ZW is the guarantor.
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [3].Zamor PJ, deLemos AS, Russo MW. Viral hepatitis and hepatocellular carcinoma: etiology and management. J Gastrointest Oncol 2017;8:229–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 2014;63:844–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol 2016;64(1 suppl):S84–101. [DOI] [PubMed] [Google Scholar]
- [6].Ge S, Huang D. Systemic therapies for hepatocellular carcinoma. Drug Discov Ther 2015;9:352–62. [DOI] [PubMed] [Google Scholar]
- [7].Qu LS, Zhang HF. Significance of viral status on prognosis of hepatitis B-related hepatocellular carcinoma after curative resection in East Asia. Hepatol Res 2016;46:40–9. [DOI] [PubMed] [Google Scholar]
- [8].Qu LS, Jin F, Huang XW, et al. High hepatitis B viral load predicts recurrence of small hepatocellular carcinoma after curative resection. J Gastrointest Surg 2010;14:1111–20. [DOI] [PubMed] [Google Scholar]
- [9].Yang X, Gao JY, Wang J, et al. The impact of anti-HBV treatment on the occurrence and recurrence of hepatocellular carcinoma: focus on Asian studies. Discov Med 2015;19:89–99. [PubMed] [Google Scholar]
- [10].Lampertico P, Agarwal K, Berg T, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;18: 30185-X. [DOI] [PubMed] [Google Scholar]
- [11].Mokdad AA, Hester CA, Singal AG, et al. Management of hepatocellular in the United States. Chin Clin Oncol 2017;6:21. [DOI] [PubMed] [Google Scholar]
- [12].Chen XP, Zhang ZW. Interpretation of “Expert consensus on the option of surgical management of hepatocelluar carcinoma”. Zhonghua Wai Ke Za Zhi 2017;55:7–10. [DOI] [PubMed] [Google Scholar]
- [13].Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg 2014;260:329–40. [DOI] [PubMed] [Google Scholar]
- [14].Jianyong L, Lunan Y, Wentao W, et al. Barcelona clinic liver cancer stage B hepatocellular carcinoma: transarterial chemoembolization or hepatic resection? Medicine 2014;93:e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim H, Ahn SW, Hong SK, et al. Survival benefit of liver resection for Barcelona Clinic Liver Cancer stage B hepatocellular carcinoma. Br J Surg 2017;104:1045–52. [DOI] [PubMed] [Google Scholar]
- [16].Bruix J, Sherman M. Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology 2005;42:1208–36. [DOI] [PubMed] [Google Scholar]
- [17].Vitale A, Morales RR, Zanus G, et al. Barcelona Clinic Liver Cancer staging and transplant survival benefit for patients with hepatocellular carcinoma: a multicentre, cohort study. Lancet Oncol 2011;12:654–62. [DOI] [PubMed] [Google Scholar]
- [18].Chen DS. Toward elimination and eradication of hepatitis B. J Gastroenterol Hepatol 2010;25:19–25. [DOI] [PubMed] [Google Scholar]
- [19].Guy J, Kelley RK, Roberts J, et al. Multidisciplinary management of hepatocellular carcinoma. Clin Gastroenterol Hepatol 2012;10:354–62. [DOI] [PubMed] [Google Scholar]
- [20].Sakamoto K, Beppu T, Hayashi H, et al. Antiviral therapy and long-term outcome for hepatitis B virus-related hepatocellular carcinoma after curative liver resection in a Japanese cohort. Anticancer Res 2015;35:1647–55. [PubMed] [Google Scholar]
- [21].Ciria R, Lopez-Cillero P, Gallardo AB, et al. Optimizing the management of patients with BCLC stage-B hepatocellular carcinoma: modern surgical resection as a feasible alternative to transarterial chemoemolization. Eur J Surg Oncol 2015;41:1153–61. [DOI] [PubMed] [Google Scholar]
- [22].Ishikawa T. Anti-viral therapy to reduce recurrence and improve survival in hepatitis B virus-related hepatocellular carcinoma. World J Gastroenterol 2013;19:8861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tarocchi M, Polvani S, Marroncini G, et al. Molecular mechanism of hepatitis B virus-induced hepatocarcinogenesis. World J Gastroenterol 2014;20:11630–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tsai HW, Lin YJ, Wu HC, et al. Resistance of ground glass hepatocytes to oral antivirals in chronic hepatitis B patients and implication for the development of hepatocellular carcinoma. Oncotarget 2016;7:27724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shim JH, Lee HC, Kim KM, et al. Efficacy of entecavir in treatment-naive patients with hepatitis B virus-related decompensated cirrhosis. J Hepatol 2010;52:176–82. [DOI] [PubMed] [Google Scholar]
- [26].Heo JY, Kim SU, Kim BK, et al. Use of Wisteria floribunda agglutinin-positive human mac-2 binding protein in assessing risk of hepatocellular carcinoma due to hepatitis B virus. Medicine 2016;95:e3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu J, Yang HI, Lee MH, et al. Spontaneous seroclearance of hepatitis B seromarkers and subsequent risk of hepatocellular carcinoma. Gut 2014;63:1648–57. [DOI] [PubMed] [Google Scholar]
- [28].Li ZQ, Hu CL, Yu P, et al. The development of hepatocarcinoma after long-term antivirus treatment of Chinese patients with chronic hepatitis B virus infection: incidence, long-term outcomes and predictive factors. Clin Res Hepatol Gastroenterol 2017;41:311–8. [DOI] [PubMed] [Google Scholar]
- [29].Kumada T, Toyoda H, Tada T, et al. Effect of nucleos(t)ide analogue therapy on hepatocarcinogenesis in chronic hepatitis B patients: a propensity score analysis. J Hepatol 2013;58:427–33. [DOI] [PubMed] [Google Scholar]
- [30].Triolo M, Della Corte C, Colombo M. Impact of HBV therapy on the incidence of hepatocellular carcinoma. Liver Int 2014;34(suppl 1):139–45. [DOI] [PubMed] [Google Scholar]
- [31].Chan AC, Chok KS, Yuen WK, et al. Impact of antiviral therapy on the survival of patients after major hepatectomy for hepatitis B virus-related hepatocellular carcinoma. Arch Surg 2011;146:675–81. [DOI] [PubMed] [Google Scholar]
- [32].Su CW, Chiou YW, Tsai YH, et al. The influence of hepatitis B viral load and pre-s deletion mutations on post-operative recurrence of hepatocellular carcinoma and the tertiary preventive effects by anti-viral therapy. PLoS One 2013;8:e66457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hiramatsu N, Yamada R, Takehara T. The suppressive effect of nucleos(t)ide analogue treatment on the incidence of hepatocellular carcinoma in chronic hepatitis B patients. J Gastroenterol Hepatol 2016;31:546–52. [DOI] [PubMed] [Google Scholar]
- [34].Kim SK, Kim SR, Imoto S, et al. Recent advances in the management of chronic hepatitis B including suppression of hepatocellular carcinoma by entecavir and interferon. Oncology 2015;89(suppl 2):60–9. [DOI] [PubMed] [Google Scholar]
- [35].Menendez-Arias L, Richman DD. Editorial overview: antivirals and resistance: advances and challenges ahead. Curr Opin Virol 2014;8:iv–vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hosaka T, Suzuki F, Kobayashi M, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology 2013;58:98–107. [DOI] [PubMed] [Google Scholar]
- [37].Thursz M, Brown A. Can antiviral therapy of chronic hepatitis B prevent the development of hepatocellular carcinoma? Gut 2011;60:1025–6. [DOI] [PubMed] [Google Scholar]
- [38].Minami M, Daimon Y, Mori K, et al. Hepatitis B virus-related insertional mutagenesis in chronic hepatitis B patients as an early drastic genetic change leading to hepatocarcinogenesis. Oncogene 2005;24:4340–8. [DOI] [PubMed] [Google Scholar]


