Abstract
Protein kinase Cζ (PKCζ) is a member of the atypical protein kinase C family. Its roles in macrophages or skin-resident keratinocytes have not been fully evaluated. In this study, we provide evidence that PKCζ mediates lipopolysaccharide (LPS)-induced tumor necrosis factor α (TNFα) gene expression in the mouse macrophage cell line, RAW264.7. TNFα has been proven to be one of the main culprits of chronic wounds and impaired acute wounds, which are characterized by excessive inflammation, enhanced proteolysis and reduced matrix deposition. Among the multiple effects of TNFα on keratinocytes, the induction of chemokines which are indispensable factors involved in the massive infiltration of various inflammatory cells into skin lesions serves as a crucial mechanism. In the present study, we found that PKCζ inhibitor or its specific siRNA inhibited the TNFα-induced upregulation in the levels of the chemokines, interleukin (IL)-8, monocyte chemotactic protein-1 (MCP-1) and intercellular cell adhesion molecule-1 (ICAM-1) in HaCaT keratinocytes. Moreover, under a disrupted inflammatory environment, activated keratinocytes can synthesize large amounts of matrix metalloproteinases (MMP), which has a negative effect on tissue remodeling. We discovered that TNFα promoted the expression of MMP9 in a PKCζ-dependent manner. Further experiments revealed that nuclear factor-κB (NF-κB) was a key downstream molecule of PKCζ. In addition, as shown in vitro, PKCζ was not involved in the TNFα-induced decrease in HaCaT cell migration and proliferation. In vivo experiments demonstrated that TNFα-induced wound closure impairment and inflammatory disorders were significantly attenuated in the PKCζ inhibitor group. On the whole, our findings suggest that PKCζ is a crucial regulator in LPS- or TNFα-induced inflammatory responses in RAW264.7 cells and HaCaT keratinocytes, and that PKCζ/NF-κB signaling may be a potential target for interventional therapy for TNFα-induced skin inflammatory injury.
Keywords: protein kinase Cζ, lipopolysaccharide, macrophage, tumor necrosis factor α, keratinocytes, chemokines, intercellular cell adhesion molecule-1, matrix metalloproteinases-9, nuclear factor-κB, chronic wounds
Introduction
Chronic wounds or impaired acute wounds severely affect the physical and mental health of patients. This non-healing wound is generally caused by bacterial infection or diabetes, as well as subsequent sustained inflammatory responses, defective matrix remodelling and impaired re-epithelialization (1). Lipopolysaccharide (LPS), a main ingredient of the Gram-negative bacterial cell wall, can trigger immune responses by stimulating macrophages to secrete inflammatory cytokines, such as interleukin (IL)-1β and tumor necrosis factor α (TNFα). TNFα is considered to be a central pathogenic factor in chronic wounds, and anti-TNFα therapy has shown promising effects in improving these injuries (2,3).
Skin-resident cells, particularly keratinocytes play an active role in cutaneous inflammation by producing various cytokines, chemokines and adhesion molecules (4). Chemokines, such as monocyte chemotactic protein-1 (MCP-1), regulated upon activation normal T cell expressed and secreted factor (RANTES), IL-8, interferon-γ-inducible protein-10 (IP-10) and intercellular adhesion molecule intercellular cell adhesion molecule-1 (ICAM-1) promote the recruitment of excessive immunocytes into skin lesions, which is a key mechanism in the deterioration of chronic wounds (3,5,6). In addition, the persistent deficiency in cell-cell and cell-matrix adhesion initiated by hyperinflammation can result in defects in tissue remodeling. Matrix metalloproteinases (MMPs) secreted by keratinocytes, fibroblasts and immune cells contribute to the degradation of extracellular matrix (ECM), and are responsible for this defect (7). Excessive MMP9 production has been strongly linked to various inflammatory skin diseases (8). Keratinocyte migration and proliferation play fundamental roles in re-epithelialization. Previous studies have reported that the capacities of migration and proliferation of keratinocytes are impaired in chronic wounds (9–12); however, the roles of TNFα are not yet completely clear.
Atypical protein kinase C (aPKC), a subfamily of atypical serine/threonine protein kinase C, requires neither Ca2+ nor diacylglycerol for optimal activity compared to conventional PKC and novel PKC subfamilies (13). PKCζ, a member of the aPKC family, is an emerging regulator of various biological processes, such as cell polarity, tumorigenesis and chemotaxis (14–19). In several cell types, PKCζ has been implicated in nuclear factor-κB (NF-κB) activation (20–23). The functions of PKCζ in LPS-stimulated macrophages and TNFα-stimulated keratinocytes have not yet been clearly examined.
The present study confirmed that PKCζ is crucial in LPS-induced TNFα expression in macrophages. Moreover, importantly, to the best of our knowledge, we demonstrate for the first time that in human HaCaT keratinocytes PKCζ regulates the TNFα-induced expression of the inflammatory related factors, IL-8, MCP-1, ICAM-1 and MMP9, which to a large degree is mediated via NF-κB activation. In addition, this study also demonstrates that TNFα at pathological concentrations (10 or 100 ng/ml) inhibits HaCaT cell migration and proliferation in vitro; however, PKCζ was not found to be involved in these processes. In vivo experiments revealed that TNFα administration to the surrounding wound tissue at the late phase of wound healing resulted in the delay of wound closure and inopportune upregulations in the levels of IL-8, MCP-1, ICAM-1 and MMP9; however, PKCζ inhibitor attenauted TNFα-initiated wound closure impairment and inflammatory disorders. Therefore, our findings provide novel insight into the prevention of the aberrant activity of skin keratinocytes induced by TNFα in chronic wounds.
Materials and methods
Cell culture
The immortalized mouse macrophage cell line, RAW264.7, and the human keratinocyte cell line, HaCaT, were obtained from the American Type Culture Collection (Manassas, Va, USA) and the China Center for Type Culture Collection (Wuhan, Hubei, China), respectively. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA). The cells were incubated in an incubator at 37°C, 5% CO2.
Cell cytotoxicity assay
The cell counting kit-8 (CCK-8) (7Sea Biotech, Shanghai, China) was used to examine the cytotoxicity according to the instructions provide by the manufacturer. The RAW264.7 cells were seeded in a 96-well plate at a density of 5.0×103 cells/well and incubated at 37°C for 12 h. The cells were then treated with fresh medium alone or with LPS (100 ng/ml; Sigma, Santa Clara, CA, USA) with or without PKCζ-specific pseudosubstrate inhibitor (1, 5 and 10 µM; Invitrogen, Carlsbad, CA, USA) for 48 h. Subseqently, 10 µl CCK-8 reagent were added to each well and the cells were cultured for a further 2 h at 37°C, 5% CO2. The absorbance was measured at 450 nm using a Tecan Infinite M200 Pro Nano Quant microplate reader (Tecan, Männedorf, Switzerland).
RNA interference
The PKCζ-specific siRNA duplexes (PKCζ-siRNA) and scramble control siRNA duplexes (scramble-siRNA) were purchased from GenePharma Co. (Shanghai, China). The sequences of these siRNAs are listed in Table I. The HaCaT cells were transfected with either PKCζ-siRNA (100 nM) or scramble-siRNA (100 nM) using Lipofectamine 2000 transfection reagent (Invitrogen) in accordance with the instructions of the manufacturer. After 6 h, the transfection mixture was removed and supplemented with fresh complete medium. The transfected cells were incubated in an incubator at 37°C, 5% CO2 for a further 20 or 40 h, and the efficiency of siRNA interference was examined by reverse transcription-quantitative PCR (RT-qPCR) or western blot analysis.
Table I.
Sequences of siRNA used in this study.
| siRNA | Sense (5′→3′) | Antisense (5′→3′) |
|---|---|---|
| PKCζ | GACACAACGAGCACUUUCUTT | AGAAAGUGCUCGUUGUGUCTT |
| Scramble | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
PKCζ, protein kinase Cζ.
RT-qPCR
The RAW264.7 cells or HaCaT cells were lysed in TRIzol reagent (Takara, Shiga, Japan) and total RNA was extracted in accordance with the instructions of the manufacturer. Total RNA (500 ng) was reverse transcribed into cDNA on the basis of the instructions of Prime Script™ RT reagent kit (Takara). The obtained cDNA was subjected to amplification using the SYBR® Premix Ex Taq™ kit (Takara) and Bio-Rad IQ5 Real-Time system (Bio-Rad, Hercules, CA, USA). The primers sequences are listed in Table II. The PCR amplification condition 4 stages: i) initial denaturation, 95°C for 30 sec; ii) denaturation, 95°C for 30 sec; iii) annealing, 58°C or 60°C for 10 sec; and iv) elongation, 72°C for 15 sec for total 38 cycles. The results were normalized against the mean Cq-values of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), namely ΔCq, and the fold increases were calculated as 2−ΔΔCq.
Table II.
Sequences of primers used in this study.
| Primers | Forward primer (5′→3′) | Reverse primer (5′→3′) |
|---|---|---|
| Mouse-TNFα | CTATCTCCAGGTTCTCTTCAA | GCAGAGAGGAGGTTGACTTTC |
| Mouse-IL-1β | GCTCATCTGGGATCCTCTCC | CCTGCCTGAAGCTCTTGTTG |
| Mouse-GAPDH | ATTGTCAGCAATGCATCCTG | ATGGACTGTGGtcATGAGCC |
| Human-PKCζ | TGCTTACATTTCCTCATCCC | TCATTCTTCTTCAACCGCAC |
| Human-MMP2 | TGAAGTATGGGAACGCCGAT | CGTACTTGCCATCCTTCTCA |
| Human-MMP9 | TCTTCCCCTTCACTTTCCTG | GCCACGAGGAACAAACTGTA |
| Human-IL-8 | TACTCCAAACCTTTCCACCC | AACTTCTCCACAACCCTCTG |
| Human-MCP-1 | GCAATCAATGCCCCAGTCA | TGCTGCTGGTGATTCTTCTATAGCT |
| Human-ICAM-1 | CACAGTCACCTATGGCAACGA | TGGCTTCGTCAGAATCACGTT |
| Human-GAPDH | CTCCTCCACCTTTGACGCTG | TCCTCTTGTGCTCTTGCTGG |
| Mouse-IL-8 | TTGCCTTGACCCTGAAGCCCCC | GGCACATCAGGTACGATCCAGGC |
| Mouse-MCP-1 | CATCCACGTGTTGGCTCA | GATCATCTTGCTGGTGAATGAGT |
| Mouse-ICAM-1 | GGCACCCAGCAGAAGTTGTT | CCTCAGTCACCTCTACCAAG |
| Mouse-MMP9 | TCTGTATGGTCGTGGCTCTA | CCTGTAATGGGCTTCCTCTATG |
TNFα, tumor necrosis factor α; IL-1β, interleukin-1β; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PKCζ, protein kinase Cζ; MMP, matrix metalloproteinase; MCP-1, monocyte chemotactic protein-1; ICAM-1, intercellular cell adhesion molecule-1.
Western blot analysis
The cells were lysed in RIPA buffer (Heart Biological Technology Co., Ltd., Xi'an, China) containing 1% aprotinin, 1% activated Na3VO4 and 1% PMSF on ice. The protein concentration was detected according to the instructions of the Pierce™ BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA). Total proteins (50 µg) were loaded onto a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel, separated by electrophoresis and transfered onto PVDF membranes (Millipore, Boston, MA, USA). The membranes were blocked with 5% skim milk at room temperature for 2 h and then incubated with rabbit anti-human PKCζ (1:1,000, #sc-216; Santa Cruz Biotechnology, Inc., CA, USA), rabbit anti-human MMP2 (1:1,000, #10373-2-AP), MMP9 (1:500, #10375-2-AP), mouse anti-human β-actin (1:1,000, #60008-1-lg) (both from China Branch of Proteintech Co., Hubei, China) and rabbit anti-human phospho-PKCζ antibodies (1:500, #9378; Cell Signaling Technology, Boston, MA, USA) at 4°C overnight. The following day, the membranes were sequentially incubated with HRP-conjugated goat anti-rabbit IgG (1:3,000, #A0208) or HRP-conjugated goat anti-mouse IgG (1:3,000, #A0216) (Beyotime, Shanghai, China) at 37°C for 1 h. Immunoreactive proteins were detected using ECL reagent (Millipore) and the FluorChem FC system (Alpha Innotech, San Leandro, CA, USA). Bands were quantitated using ImageJ software.
Nuclear and cytoplasmic protein extraction
The cells were seeded in a 6-well plate and incubated at 37°C for 24 h. The cells were pre-treated with or without the NF-κB inhibitor, BAY11-7082 (5 µM; Selleck, Houston, TX, USA), for 2 h or PKCζ I (5 µM; Invitrogen) for 3 h and then stimulated with TNFα (rhTNFα, 10 ng/ml; Shanghai Bioleaf Biotech Co., Ltd., Shanghai, China) for 2 h in the presence of BAY11-7082 or PKCζ I. The nuclear proteins were isolated on the basis of the intructions of a commercial nuclear and cytoplasmic protein extraction kit (Beyotime). Separated nuclear proteins were analyzed by immunoblotting. The internal reference protein of nuclear protein was histone H3. The primary antibodies included rabbit anti-human NF-κB-p65 antibody (1:500, #sc-372) and goat anti-human histone H3 antibody (1:500, #sc-8654) (Santa Cruz Biotechnology, Inc.). The secondary antibodies included HRP-conjugated goat anti-rabbit IgG (1:1,000, #A0208) and donkey anti-goat IgG (1:1,000, #A0108) (Beyotime).
NF-κB immunofluorescence analysis
All operations were executed in accordance with the instructions of the NF-κB activation, Nuclear Translocation assay kit (Beyotime). Cells growing on coverslip were fixed with 4% paraformaldehyde for 20 min, and then incubated with 5% BSA for 30 min at room temperature to suppress non-specific binding of IgG. The cells were then incubated with rabbit anti-human NF-κB-p65 antibody overnight at 4°C. The following day, the NF-κB-p65 antibody was removed and the cells were sequentially incubated with anti-rabbit Cy3 IgG at room temperature for 1 h. The nucleus was stained with DAPI. Images were captured using a FSX100 microscope (Olympus, Tokyo, Japan).
Cell proliferation assay
Cell proliferation was evaluated by CCK-8 assay (7Sea Biotech) according to the instructions provided by the manufacturer. Briefly, the cells were seeded in a 96-well plate at a density of 4.0×103 cells/well and incubated at 37°C for 24 h. The cells were then treated with or without PKCζ-specific pseudosubstrate inhibitor (10 µM; Invitrogen), After 3 h, the inhibitor mixture was removed and replaced with fresh medium alone or with TNFα (rhTNFα, 10 or 100 ng/ml; Shanghai Bioleaf Biotech Co., Ltd.) for 24, 48 and 72 h. At the indicated time point, the medium was removed and supplemented with the medium containing CCK-8 reagent (10 µl/well) and the cells were cultured for a further 1–1.5 h at 37°C, 5% CO2. Subsequently, the absorbance was measured at 450 nm using a Tecan Infinite M200 Pro Nano Quant micro-plate reader (Tecan).
Scratch wound healing assay
The HaCaT cells were seeded in 35-mm culture dishes in DMEM supplemented with 10% FBS (both from Gibco) at 37°C, 5% CO2 for 24 h, until full confluence. Subsequently, 10 µg/ml mitomycin C (Invitrogen) was added for 1 h to completely inhibit cell proliferation. A scratch of 500 µm in width was created on the confluent cells with a 10 µl pipette tip. The cells were washed 3 times with phosphate-buffered saline (PBS) and pre-treated with or without PKCζ inhibitor (10 µM; Invitrogen) for 3 h, then incubated in serum-free DMEM alone (control group), or serum-free DMEM containing TNFα (10 or 100 ng/ml; Bioleaf) at 37°C, 5% CO2 for 24 h. The cells were photographed under a microscope and the gap width of scratch was measured using Image Pro Plus software. The cell migration distance was the difference value between the gap width at 0 and 24 h. The migration distance at control group was set as 1.
Detection of the activity of PKCζ in vivo
Male 6-week old BALB/c mice (n=40) were obtained from The Center of Experimental Animal, the Fourth Military Medical University. The experiment was repeated 3 times. All animal experiments were performed in accordance with the guidelines from the Administration of Animal Experiments for Medical Research Purposes issued by the Ministry of Health of China, which is in accordance with the principle of ARRIVE. The protocol was approved by the Animal Experiment Administration Committee of the Fourth Military Medical University. The mice were randomly divided into 2 groups (n=8) as follows: the PBS control group and TNFα group. To examine the differences in the phosphorylation level of PKCζ between the PBS- and TNFα-treated wounds, a 1.0×1.0 cm full-thickness wound was created on the back of each BALB/c mouse following anesthetization by an intraperitoneal injection of 1% pentobarbital sodium (40 mg/kg). Subsequently, 100 µl PBS or 100 µl TNFα (200 ng, dissolved in PBS) was injected into the tissue around the wound, and after 2 h, the samples of the wound-edge skin (WES) and normal skin (NS) in each mouse were collected for the examination of the levels of PKCζ and phosphorylated PKCζ by western blot analysis.
In vivo wound closure assay
The BALB/c mice were randomly divided into 3 groups (n=8) as follows: the PBS control group, TNFα group and TNFα + PKCζ inhibitor group. Following anesthetization, a 1.0×1.0 cm full-thickness wound was created on the back of each BALB/c mouse. Subsequenlty, 100 µl PBS, 100 µl TNFα solution (rmTNFα, 50 ng, dissolved in PBS; Bioleaf) or 100 µl TNFα solution + PKCζ inhibitor (20 µM) was injected into the skin tissue around the wound each day from day 6 post-incision (days 6, 7, 8, 9, 10, 11 and 12 post-incision). The wound area of each mouse was digitally photographed and measured using Photoshop software or the skin tissue samples of the wound edges in each mouse were collected for mRNA extraction and RT-qPCR analysis on days 7, 9 and 13 post-wounding.
Statistical analysis
All experiments were repeated at least 3 times, and the results are the means ± SD. The analysis of statistically significant differences between groups was performed by one-way analysis of variance (ANOVA) with the Student's t-test or the Mann-Whitney U test using SPSS 17.0 software. A value of p<0.05 was considered to indicate a statistically significant difference.
Results
PKCζ mediates LPS-induced inflammatory responses in RAW264.7 cells
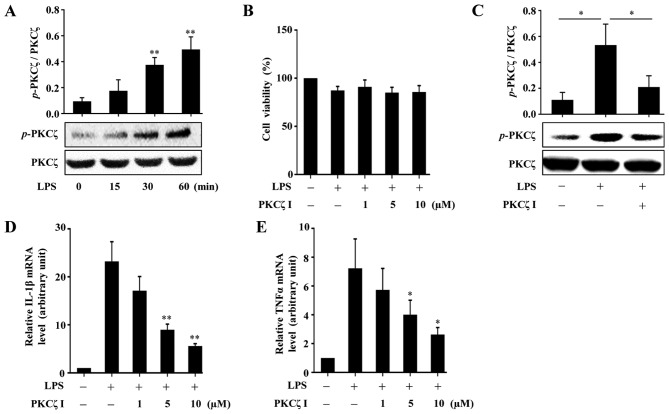
A previous study reported that PKCζ activity is required for LPS-induced IL-1β production in RAW264.7 cells (22); however, whether PKCζ mediates the expression of another important inflammatory factor, namely TNFα, has not yet been examined, at least to the best of our knowledge. PKCζ can be activated through phosphorylating its threonine 410 site. In the present study, we validated that stimulation with LPS (100 ng/ml) resulted in an increase in the phosphorylation level of PKCζ in the RAW264.7 cells (Fig. 1A). To measure the cytotoxicity of LPS or PKCζ-specific pseudosubstrate inhibitor (PKCζ I) in the RAW264.7 cells, CCK-8 assay was performed. The results revealed that both LPS at a concentration of 100 ng/ml and PKCζ I up to 10 µM did not damage the viability of the RAW264.7 cells (Fig. 1B). Pre-treatment of the cells with PKCζ I inhibited the LPS-induced phosphorylation of PKCζ (Fig. 1C) and suppressed the LPS-induced increase in the mRNA expression levels of IL-1β and TNFα (Fig. 1D and E).
Figure 1.
The roles of protein kinase Cζ (PKCζ) in lipopolysaccharide (LPS)-induced inflammatory responses in RAW264.7 macrophages. (A) Representative immunoblots assessing the changes in the levels of phosphorylated PKCζ in RAW264.7 cells following treatment with 100 ng/ml LPS at indicated time points (0, 15, 30 and 60 min). (B) CCK-8 assay assessing the effects of LPS (100 ng/ml) alone or with PKCζ specific pseudosubstrate inhibitor (PKCζ I) (1, 5 or 10 µM) on the viability of RAW264.7 cells. Data represent means ± SD of 3 independent experiments, the value in LPS-free group arbitrarily set as 100%. (C) Representative immunoblots showing the effects of PKCζ I (10 µM) on LPS-induced PKCζ phosphorylation. (D and E) RT-qPCR analysis showing the effects of PKCζ I on LPS-induced mRNA level changes of interleukin-1β (IL-1β) or tumor necrosis factor α (TNFα). Each bar represent the means ± SD of 3 independent experiments, and normalized to corresponding total PKCζ protein level (A and C) or GAPDH mRNA level with the value in the LPS-free group set as 1 arbitrarily (D and E); *p<0.05 and **p<0.01 vs. the value at 0 min (A) or the value in corresponding LPS alone group (C–E).
TNFα promotes the activation of PKCζ in HaCaT keratinocytes
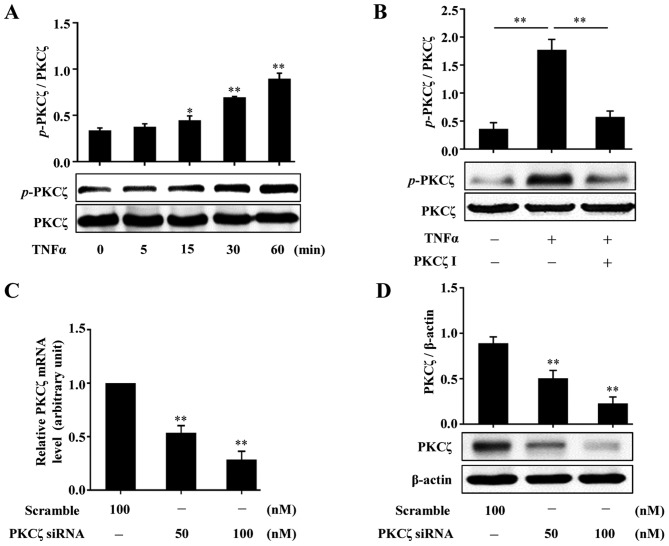
To examine whether PKCζ is involed in the effects of TNFα on HaCaT keratinocytes, we firstly examined whether TNFα stimulates the activation of PKCζ. Following treatment with TNFα (10 ng/ml) and at the indicated time points (0, 5, 15, 30 and 60 min), the HaCaT cells were lysed and the lysates were assessed by western blot analysis. The results revealed that exposure to TNFα resulted in increased phosphorylation levels of PKCζ, which was firstly observed at 15 min and reached peak levels at 60 min (Fig. 2A). We then found that pre-treatment of the cells with PKCζ I (10 µM) for 3 h distinctly suppressed the TNFα-induced PKCζ phosphorylation (Fig. 2B). In addition, the effects of PKCζ-specific small interfering RNA (PKCζ-siRNA) were examined by RT-qPCR and western blot analysis. The results revealed that the mRNA and protein expression levels of PKCζ were all effectively suppressed (Fig. 2C and D).
Figure 2.
Effects of tumor necrosis factor α (TNFα) on the activation of protein kinase Cζ (PKCζ) in HaCaT cells. (A) Representative immunoblots showing the changes in the phosphorylation levels of PKCζ in HaCaT cells stimulated with 10 ng/ml TNFα at indicated time points (0, 5, 15, 30 and 60 min), respectively. (B) Representative immunoblots showing the effects of PKCζ I pre-treatment on TNFα-induced PKCζ phosphorylation. (C and D) RT-qPCR (C) or immunoblotting (D) assessing the effects of PKCζ specific interfering RNA (PKCζ-siRNA) on the knockdown of PKCζ at the mRNA and protein levels. Bars represent the means ± SD of 3 independent experiments, and normalized to corresponding total PKCζ protein level (A and B), glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA level with the value in the scramble group set as 1 arbitrarily (C) or β-actin protein level (D). *p<0.05 and **p<0.01 vs. the value at 0 min (A), in TNFα alone group (B), or in scramble group (C and D).
PKCζ is involved in TNFα-induced inflammatory responses in HaCaT cells
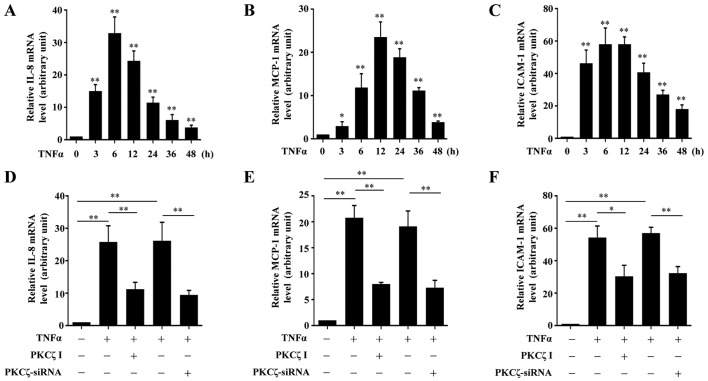
It is known that the chemokines, MCP-1 and IL-8, and the adhesion molecule, ICAM-1, are crucial in the pathogenesis of inflammatory disorders in chronic wounds. Thus, to determine whether PKCζ is involved in the TNFα-induced inflammatory responses in HaCaT cells, we examined the effects of PKCζ I on the mRNA expression levels of IL-8, MCP-1 and ICAM-1. Exposure to TNFα led to an increase in the mRNA expression levels of IL-8, MCP-1 and ICAM-1 over time which respectively peaked at 6, 12 and 6 h post-TNFα treatment, and then gradually declined, but remained at significantly high levels at 48 h (Fig. 3A–C). The cells were pre-treated with PKCζ I for 3 h in advance, and then stimulated with TNFα for 12 h, which largely inhibited the TNFα-induced upregulation of IL-8, MCP-1 and ICAM-1. The silencing of PKCζ by transfection with siRNA for 6 h also led to similar results (Fig. 3D–F).
Figure 3.
The roles of protein kinase Cζ (PKCζ) in tumor necrosis factor α (TNFα)-induced expression of interleukin-1β (IL-8), monocyte chemotactic protein-1 (MCP-1) and adhesion molecule intercellular cell adhesion molecule-1 (ICAM-1) in HaCaT cells. (A–C) The mRNA levels of (A) IL-8, (B) MCP-1 and (C) ICAM-1 in HaCaT cells exposed to TNFα (10 ng/ml) at indicated time points (0, 3, 6, 12, 24, 36 and 48 h) were assessed by RT-qPCR assays. (D–F) RT-qPCR assays showing the effects of PKCζ I (10 µM) or PKCζ-siRNA (100 nM) on the TNFα-induced upregulation of (D) IL-8, (E) MCP-1 and (F) ICAM-1. Bars represent the means ± SD of 3 independent experiments, and normalized to the GAPDH mRNA level with the value in the TNFα-free treatment group set as 1 arbitrarily. *p<0.05 and **p<0.01 vs. the value at 0 h (A–C).
PKCζ mediates the TNFα-induced upregulation of MMP9 in HaCaT cells
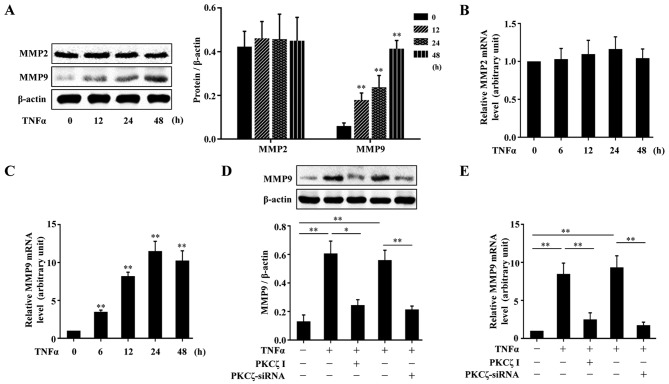
MMPs, are a crucial class of protease, and have been reported to be abnormally upregulated in impaired wound healing (7,8). We firstly examined the effects of TNFα on the expressions of MMP2 and MMP9 in HaCaT cells. The cells were treated with TNFα for various periods of time (0, 12, 24 and 48 h), and the cells were lysed and the lysates were assessed by western blot analysis. The results revealed that TNFα had no significant effect on the protein expression of MMP2; however, MMP9 protein expression was indeed elevated by exposure to TNFα (Fig. 4A). The analysis of MMP2 and MMP9 mRNA expression levels by RT-qPCR also revealed similar results (Fig. 4B and C). To explore the role of PKCζ in the TNFα-induced changes in the levels of MMP9 in the HaCaT cells, we used PKCζ I or PKCζ siRNA to disrupt the normal function of PKCζ. The results revealed that the TNFα-induced upregulation of MMP9 at both the protein and mRNA level tightly depended on the activity of PKCζ, as treatment with PKCζ I or PKCζ siRNA decreased MMP9 expression (Fig. 4D–E).
Figure 4.
Effect of tumor necrosis factor α (TNFα) on the expression of matrix metalloproteinase in HaCaT cells and the role of protein kinase Cζ (PKCζ). (A) Representative immunoblots showing the protein level changes of matrix metalloproteinase (MMP)2 and MMP9 in HaCaT cells exposed to TNFα (10 ng/ml). (B and C) RT-qPCR analysis showing the changes in the mRNA levels of MMP2 and MMP9 in HaCaT cells stimulated with TNFα. (D and E) Immunoblotting (D) or RT-qPCR (E) assessing the effect of PKCζ I or PKCζ-siRNA on the TNFα-induced upregulation of MMP9 at protein or mRNA level. Bars represent the means ± SD of 3 independent experiments, and normalized to the β-actin protein level (A and D) or GAPDH mRNA level with the value in TNFα-free group set as 1 arbitrarily (B, C and E). *p<0.05 and **p<0.01 vs. the value at 0 h (A–C).
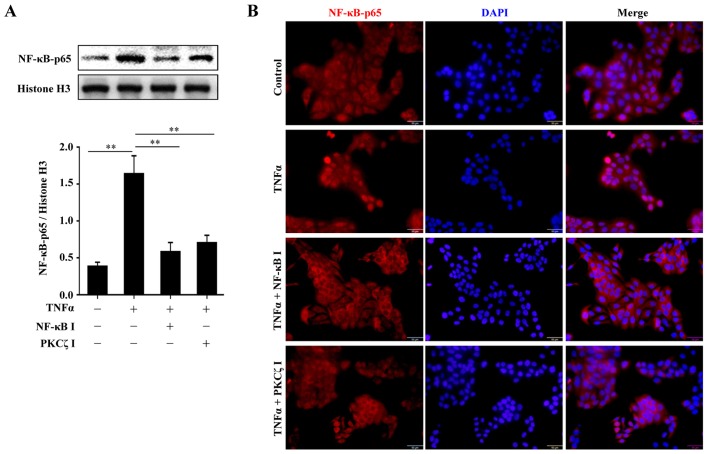
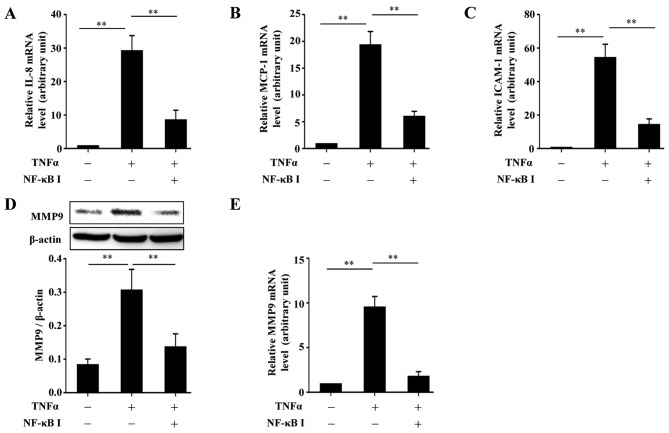
Inhibition of the activity of PKCζ impairs the TNFα-induced nuclear translocation of NF-κB-p65 that is required for the upregulation of MCP-1, IL-8, ICAM-1 and MMP9 following stimulation with TNFα
From the above-mentioned results, we found that PKCζ regulated the expression of MCP-1, IL-8, ICAM-1 and MMP9 beginning from the transcriptional level. Moreover, several studies have suggested that PKCζ is likely to be an upstream regulator of NF-κB, which is an important transcription factor involved in the regulation of many biological processes (20–23). Thus, we hypothesized that the PKCζ-mediated inflammatory responses and MMP9 expression in HaCaT cells may depend on the activation of NF-κB. The cells were pre-treated with the NF-κB inhibitor, BAY11-7082, for 2 h or PKCζ I for 3 h, and then stimulated with TNFα for 2 h in the presence of BAY11-7082 or PKCζ I. The nuclear transport of p65, a key subunit of NF-κB, was assessed by western blot analysis or immunofluorescence. The results revealed that following TNFα stimulation, there was a distinct nuclear translocation of p65; however, this nuclear import was prevented by PKCζ I, which was in accordance with the function of BAY11-7082 (Fig. 5). Further experiments confirmed the requirement of NF-κB in the TNFα-induced upregulation of MCP-1, IL-8, ICAM-1 and MMP9 by inhibiting the function of NF-κB with BAY11-7082. The use of BAY11-7082 decreased the levels of MCP-1, IL-8, ICAM-1 and MMP9 induced by TNFα (Fig. 6).
Figure 5.
Involvement of protein kinase Cζ (PKCζ) in tumor necrosis factor α (TNFα)-induced NF-κB-p65 nuclear translocation. (A) Representative immunoblots showing the effects of NF-κB inhibitor BAY11-7082 and PKCζ I on TNFα-induced NF-κB-p65 nuclear import. (B) The localization of NF-κB-p65 in HaCaT cells was analyzed by immunofluorescence. Bars represent the means ± SD of 3 independent experiments, and normalized to histone H3 protein level. **p<0.01. Scale bar, 50 µm.
Figure 6.
The role of NF-κB in tumor necrosis factor α (TNFα)-induced expression of interleukin-1β (IL-8), monocyte chemotactic protein-1 (MCP-1), intercellular cell adhesion molecule-1 (ICAM-1) and MMP9 in HaCaT cells. (A–C) RT-qPCR analysis showing the effects of BAY11-7082 on the TNFα-induced changes in the mRNA levels of (A) IL-8, (B) MCP-1 and (C) ICAM-1 in HaCaT cells. (D and E) Immunoblotting (D) or RT-qPCR (E) assays showing the effect of BAY11-7082 on the TNFα-induced epression of MMP9 at protein or mRNA level in HaCaT cells. All the bars represent the means ± SD of 3 independent experiments, and normalized to the GAPDH mRNA level with the value in the TNFα-free group set as 1 arbitrarily (A–C and E) or to β-actin level (D). **p<0.01.
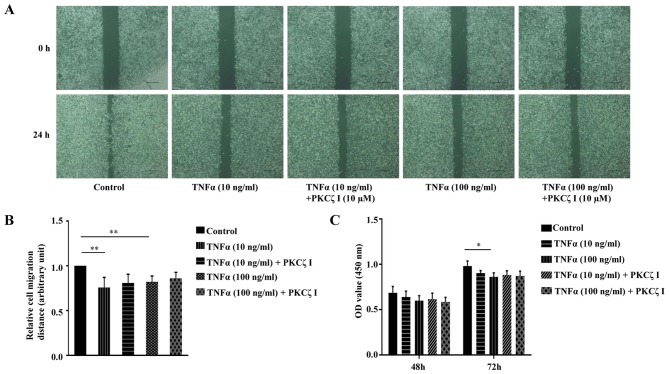
PKCζ does not significantly affect TNFα-weakened HaCaT keratinocyte migration and proliferation in vitro
Keratinocyte migration and proliferation are important to the rate of wound closure. We thus examined the effect of TNFα and PKCζ I on HaCaT cell migration using a scratch wound healing model. The cells were treated with 10 µg/ml mitomycin C for 1 h to inhibit cell proliferation. A scratch was made on fully confluent cells. Subsequently, following pre-treatment with or without PKCζ I for 3 h, the cells were stimulated with TNFα at pathological concentrations (10 or 100 ng/ml) for 24 h. The results revealed that exposure to TNFα markedly decreased the rate of wound healing and the inhibition of the function of PKCζ by PKCζ I did not significantly attenuate the TNFα-induced decrease in keratinocyte migration (Fig. 7A and B). CCK-8 assay was then used to examine the effect of TNFα and PKCζ on HaCaT cell proliferation. The absorbance at 450 nm was directly proportional to the cell proliferation capacity. The results revealed that pathological concentrations of TNFα caused a small degree of inhibition on cell proliferation at 48 h; however, this difference was not statistically significant. Until 72 h, exposure to 100 ng/ml TNFα inhibited cell proliferation by approximately 11.6%, which was statistically significant (Fig. 7C). We also found that PKCζ I pre-treatment did not signifincantly affect the TNFα-induced impairment of keratinocyte proliferation (Fig. 7C).
Figure 7.
Effects of tumor necrosis factor α (TNFα) on the ability of migration and proliferation of HaCaT cells in vitro and the role of protein kinase Cζ (PKCζ) in these processes. (A) Representative images of the gap areas at 0 or 24 h after TNFα (10 or 100 ng/ml) treatment in the absence or presence of PKCζ I in scratch wound healing assay. Scale bar, 200 µm. (B) Histogram showing the relative cell migration distance. The migration distance was the difference value between the gap width at 0 and 24 h and the value at control group was set as 1 arbitrarily. (C) CCK-8 assay showing the proliferation of HaCaT cells after TNFα (10 or 100 ng/ml) treatment with or without PKCζ I. The absorbance at 450 nm is in direct proportion to the cell proliferation ability. Bars represent the means ± SD of 3 independent experiments. *p<0.05 and **p<0.01.
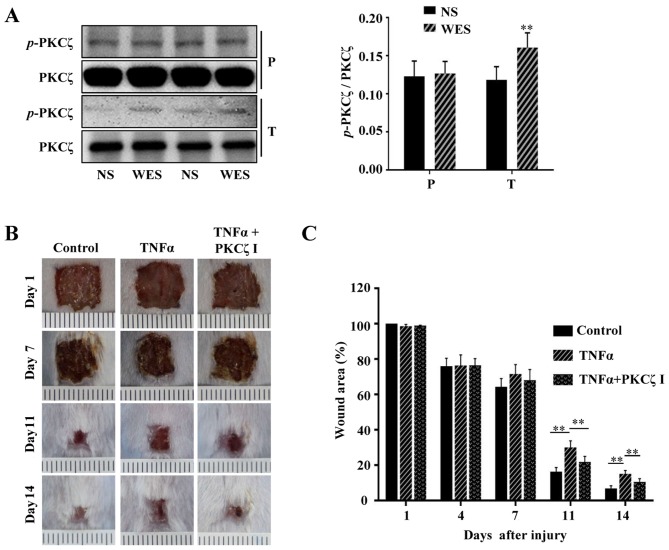
PKCζ inhibition attenuates the TNFα-induced wound closure delay and inflammatory disorders in vivo
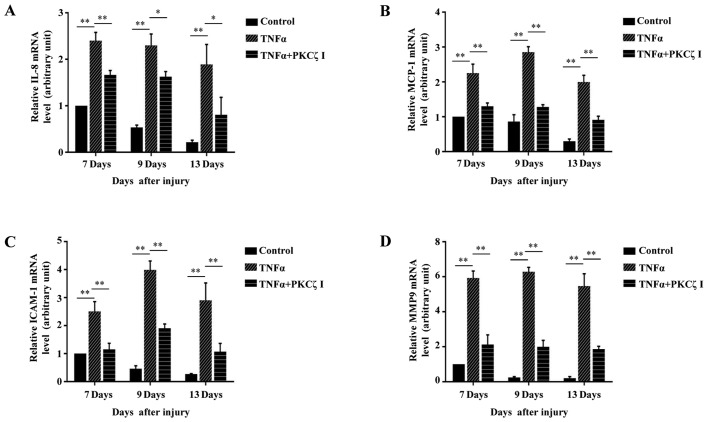
Previous studies have reported that from the 5th day after normal acute wounding, the mRNA levels of 2 pro-inflammatory cytokines, IL-1β and TNFα, and the chemokine, MCP-1, in wound tissues are declined and cannot be detected on day 13 post-wounding; however, in the wounds of diabetic mice, the mRNA levels of IL-1β, TNFα and MCP-1 are still strongly elevated and sustained during the late phase (days 7–13 after wounding) of the wound repair process (5,24). It has become a consensus that sustained inflammation triggers the prolonged infiltration of immune cells at the late stage of cutaneous wound repair and can cause defects in wound healing. Furthermore, a number of studies have confirmed that the local injection of potent pro-inflammatory cytokines, IL-1α or TNFα or IFN-γ, in the skin of various animals, such as mice, dogs, rats, rabbits and human volunteers can cause significant inflammatory responses and the rapid infiltration of immunocytes (25–30). In the present study, we locally injected PBS or TNFα into the wound edges of BALB/c mice, and after 2 h we found that compared with the normal cutaneous tissue, the phosphorylation of PKCζ was increased in the wound tissue treated with TNFα (Fig. 8A). To explore whether prolonged TNFα exposure can result in wound closure delays in mice and whether PKCζ is involved in this process, 100 µl PBS, 100 µl TNFα solution (50 ng, dissolved in PBS) or 100 µl TNFα solution + PKCζ inhibitor (20 µM) were injected into the skin tissue around the wound each day on days 6–12 post-wounding. The results revealed that the TNFα injection at the late phase of wound closure caused a delay in wound repair; however, the administration of PKCζ inhibitor led to a favourable improvement, attenuating this impairment (Fig. 8B and C). Our in vitro experiments revealed that PKCζ played an indispensable role in TNFα-induced inflammatory responses in keratinocytes. Total RNA was extracted from the wound tissues of the PBS, TNFα and TNFα + PKCζ I-treated mice at different intervals (days 7, 9 and 13 after wounding) for RT-qPCR analysis. We observed a gradual derease in the levels of IL-8, MCP-1, ICAM-1 and MMP9 to very low levels on day 13 post-wounding in the PBS control mice. However, in the TNFα-treated mice, until day 13 post-wounding, the expression levels of these molecules remained at markedly high levels, which may be one of the causes that resulted in defecs in wound closure. However, as expected, PKCζ inhibition markedly inhibited the TNFα-induced upregulation in the levels of IL-8, MCP-1, ICAM-1 and MMP9 (Fig. 9).
Figure 8.
The activity of protein kinase Cζ (PKCζ) in tumor necrosis factor α (TNFα)-treated wounds and effects of PKCζ inhibitor on the attenuation of prolonged TNFα environment-induced skin wound closure delay. (A) Representative immunoblots showing the phosphorylation levels of PKCζ in paired tissue samples of the normal skin (NS) and PBS or TNFα-treated wound edge skin (WES). P, PBS-treated mouse; T, TNFα-treated mouse. (B and C) Representative images of the wound areas in PBS group (control), TNFα group and TNFα+PKCζ inhibitor (PKCζ I) group on days 1, 7, 11 and 14 post-incision. The wound closure rate was the ratio of the remanent wound area on the indicated days and the original wound area on day 1 post-incision. Bars represent the means ± SD of 3 independent experiments, n=8. **p<0.01.
Figure 9.
Roles of protein kinase Cζ (PKCζ) in prolonged tumor necrosis factor α (TNFα) environment-induced upregulation of interleukin-1β (IL-8), monocyte chemotactic protein-1 (MCP-1), intercellular cell adhesion molecule-1 (ICAM-1) and MMP9. (A–D) RT-qPCR analysis showing the effects of PKCζ inhibitor (PKCζ I) on the TNFα-induced increased mRNA levels of (A) IL-8, (B) MCP-1 and (C) ICAM-1 and (D) MMP9 in wounds edges at different days of the late phase of wound healing (days 7, 9 and 13 after wounding). Bars represent the means ± SD of 3 independent experiments, and normalized to the GAPDH mRNA level. The value on day 7 in control group was set as 1 arbitrarily. *p<0.05 and **p<0.01.
Discussion
A severely enhanced and prolonged TNFα environment in chronic wounds, such as diabetic foot ulcers can trigger abnormally excessive inflammatory responses, which leads to severe defects in tissue remodeling and re-epithelialization (1). This notoriously non-healing wound is a threat to human health and the improvement of this injury is challenging.
Previous studies have suggested that the roles of PKCζ in different biological processes depend on cell types, milieu and specific binding partners (31,32). Bacterial LPS can powerfully activate the innate immune system of the body (33). It has been shown that PKCζ activity is required for LPS-stimulated IL-1β gene expression in RAW264.7 cells (22). This study demonstrated that not only IL-1β, but also the expression of TNFα was regulated by PKCζ activity in LPS-stimulated RAW264.7 cells (Fig. 1).
TNFα is considered to be one of the main culprits in non-healing wounds. Keratinocytes exposed to TNFα show inflammatory related functions other than maintaining epithelization. In the inflammatory epidermis, the chemokines, IL-8 and MCP-1, and the intercellular adhesion molecule-1 (ICAM-1), play important roles in mediating the interaction between immune cells and keratinocytes (34–36). These infiltrating immune cells synthesize more and more inflammatory cytokines and chemokines to further lead to the amplification of inflammation, which contributes to the pathogenesis of chronic wounds. Therefore, the in-depth mechanisms of the effects of TNFα on keratinocytes are worthy of exploration. Previous studies have indicated that PKCζ is an important regulator of TNFα signaling via the NF-κB and MAPK signaling pathways in retinal endothelial cells and human umbilical vein endothelial cells (23,37,38); however, the function of PKCζ in human keratinocytes has not been fully investigated. Moreover, a previous study indicated that PKCζ expression was relatively increased and that it was involved in the upregulation of CD1d in the lesions of psoriasis, which is another chronic inflammatory skin disorder characterized by abnormal TNFα stimulation (39). This study demonstrated that TNFα enhanced the phosphorylation level (Thr410) of PKCζ in a time-dependent manner in HaCaT keratinocytes (Fig. 2A), which led us to explore whether PKCζ plays a role in TNFα-induced inflammatory responses. PKCζ-specific pseudosubstrate peptide inhibitor (PKCζ I) or siRNA was used to block the activaty of PKCζ (Fig. 2B) or to silence its expression (Fig. 2C and D). The present study demonstrated that the mRNA expression levels of IL-8, MCP-1 and ICAM-1 in the HaCaT keratinocytes were all elevated by TNFα stimulation, and peaked respectively at 6, 12 and 6 h post-TNFα treatment and gradually reduced with time (Fig. 3A–C). Pre-treatment with PKCζ I or PKCζ-siRNA significantly prevented the TNFα-induced upregulation in the levels of IL-8, MCP-1 and ICAM-1 (Fig. 3D–F). These results provide new insight, indicating that PKCζ I is a positive regulator of the pro-inflammatory effects of TNFα on keratinocytes.
Compared with MMP2, MMP9 is poorly expressed in normal skin (40). In acute wounds, MMP9 is rapidly increased as an early response to injury, and then is rapidly decreased to the basic levels (29). The tight regulation and control of MMP9 are important since the persistent and high expression of MMP9 initiated by excessive inflammation can lead to a sustained deficiency in cell-cell and cell-matrix adhesion, which is the subsequent cause of non-healing wounds (8,41,42). Moreover, active MMP9 has been reported that to cleave and activate IL-8 (43,44), which may result in the further dysregulation of inflammation. Our results revealed that the exposure of HaCaT cells to TNFα incured a time-dependent upregulation in the levels of MMP9; however, MMP2 was was not affected (Fig. 4A–C). PKCζ has been reported to be involved in the regulation of MMP9 in rabbit smooth muscle cells (45). Therefore, we speculated that PKCζ may be an important mediater in the TNFα-induced expression of MMP9 in keratinocytes. As expected, inhibiting the function of PKCζ using an inhibitor or siRNA significantly suppressed the increased expression of MMP9 stimulated by TNFα at both the mRNA and protein level (Fig. 4D and E).
In previous studies PKCζ was reported to be involved in NF-κB activation by affecting the Ser311 site phosphorylation or nuclear translocation of p65, a key subunit of the NF-κB complex (20–23). However, the association between PKCζ and NF-κB has not been explored in human keratinocytes, at least to the best of our knowledge. Our results of western blot analysis of nucleoproteins demonstrated that in HaCaT cells, PKCζ inhibitor exerted similar effects to the NF-κB inhibitor, BAY11-7082, by partly preventing the TNFα-induced nuclear import of p65 (Fig. 5A), and the results from immunofluorescence assay also confirmed the above conclusion (Fig. 5B). Further experiments indicated that BAY11-7082 inhibited the TNFα-induced increase in the expression of IL-8, MCP-1, ICAM-1 and MMP9 (Fig. 6A–D). Therefore, our results indicated that in HaCaT keratinocytes, PKCζ regulates the expression of IL-8, MCP-1, ICAM-1 and MMP9 by influencing NF-κB activation.
Keratinocytes require the transition from a quiescent phenotype to an actively migratory and proliferative phenotype during wound healing. Accumulating evidence has indicated that keratinocytes at the diabetic wound edges have an impaired migration and proliferative activity (9,10). Our scratch wound assay demonstrated that TNFα at pathological concentrations significantly inhibited HaCaT cell migration (Fig. 7A and B), which was consistent with the results of a previous study by Zhang et al (10). PKCζ inhibitor faintly improved this inhibition of cell migration; however, its effect was not significant (Fig. 7A and B). TNFα has been reported to actively participate in the promotion of apoptosis and inhibition of proliferation in many other epithelial cell types (46,47). Our CCK-8 proliferation assay revealed that exposure to 100 ng/ml TNFα for 72 h inhibited the proliferation of HaCaT cells; however, this inhibitory effect was not strong, and thus HaCaT cells were not very sensitive to the TNFα-induced inhibition of proliferation as other epithelial cell types (Fig. 7C). Of note, we found that the presence of PKCζ I appeared to faintly increase this sensitivity (Fig. 7C). This may be due to the fact that TNFα/PKCζ/NF-κB can also induce the expression of some anti-apoptotic proteins (48).
In the present study, PBS or TNFα was locally administered to the wound edges of BALB/c mice, and we found that after 2 h, TNFα treatment effectively upregulated the phosphorylation level of PKCζ in wound tissue (Fig. 8A). Previous studies have shown that in chronic wounds, the expression of TNFα does not return to the basic level, but maintains a persistent high level at the late stage (5,24). Local injections of inflammatory cytokines in the skin can induce rapid inflammatory reaction (25–30), thus mimicking the environment of various inflammatory skin disease. To further examine whether the prolonged presence of TNFα at the late phase of wound closure influences the rate of wound healing, exogenous TNFα was injected into the tissue around the wound on day 6–12 post-wounding. We demonstrated that the administration of TNFα markedly inhibited wound closure (Fig. 8B and C) and stimulated the sustained high expression of IL-8, MCP-1, ICAM-1 and MMP9 in wounds (Fig. 9). The concomitant administration of PKCζ I significantly attenuated this non-healing condition (Fig. 8B and C) and resulted in the attenuation of the TNFα-induced upregulation of the above-mentioned molecules (Fig. 9). These findings suggest that PKCζ is involved in TNFα-triggered inflammatory disorders in unhealed wounds. In the future, in order to elucidate the mechanisms through which PKCζ regulates chronic wound healing, further investigations are clearly warranted.
In conclusion, to the best of our knowledge, this study, for the first time indicates that PKCζ effectively regulates TNFα-induced inflammatory responses and MMP9 expression in vitro and in vivo, which may provide a promising target for researching the interventional therapy of chronic inflammatory skin defects.
Abbreviations
- PKCζ
protein kinase Cζ
- IL-1β
interleukin-1β
- TNFα
tumor necrosis factor α
- LPS
lipopolysaccharide
- IL-8
interleukin-8
- MCP-1
monocyte chemotactic protein-1
- RANTES
regulated upon activation normal T cell expressed and secreted factor
- IP-10
interferon-γ-inducible protein-10
- ICAM-1
intercellular cell adhesion molecule-1
- MMP2
matrix metalloproteinase 2
- MMP9
matrix metalloproteinase 9
- NF-κB
nuclear factor-κB
References
- 1.Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173:370–378. doi: 10.1111/bjd.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Streit M, Beleznay Z, Braathen LR. Topical application of the tumour necrosis factor-alpha antibody infliximab improves healing of chronic wounds. Int Wound J. 2006;3:171–179. doi: 10.1111/j.1742-481X.2006.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu F, Zhang C, Graves DT. Abnormal cell responses and role of TNF-α in impaired diabetic wound healing. BioMed Res Int. 2013;2013:754802. doi: 10.1155/2013/754802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker JN, Mitra RS, Griffiths CE, Dixit VM, Nickoloff BJ. Keratinocytes as initiators of inflammation. Lancet. 1991;337:211–214. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- 5.Wetzler C, Kämpfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: Prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol. 2000;115:245–253. doi: 10.1046/j.1523-1747.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- 6.Lan CC, Wu CS, Huang SM, Wu IH, Chen GS. High-glucose environment enhanced oxidative stress and increased interleukin-8 secretion from keratinocytes: New insights into impaired diabetic wound healing. Diabetes. 2013;62:2530–2538. doi: 10.2337/db12-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, Murphy G, Schultz G. Analysis of the acute and chronic wound environments: The role of proteases and their inhibitors. Wound Repair Regen. 1999;7:442–452. doi: 10.1046/j.1524-475X.1999.00442.x. [DOI] [PubMed] [Google Scholar]
- 8.Rayment EA, Upton Z, Shooter GK. Increased matrix metal-loproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol. 2008;158:951–961. doi: 10.1111/j.1365-2133.2008.08462.x. [DOI] [PubMed] [Google Scholar]
- 9.Lan CC, Liu IH, Fang AH, Wen CH, Wu CS. Hyperglycaemic conditions decrease cultured keratinocyte mobility: Implications for impaired wound healing in patients with diabetes. Br J Dermatol. 2008;159:1103–1115. doi: 10.1111/j.1365-2133.2008.08789.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C, Ponugoti B, Tian C, Xu F, Tarapore R, Batres A, Alsadun S, Lim J, Dong G, Graves DT. FOXO1 differentially regulates both normal and diabetic wound healing. J Cell Biol. 2015;209:289–303. doi: 10.1083/jcb.201409032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galkowska H, Olszewsk WL, Wojewodzka U, Mijal J, Filipiuk E. Expression of apoptosis- and cell cycle-related proteins in epidermis of venous leg and diabetic foot ulcers. Surgery. 2003;134:213–220. doi: 10.1067/msy.2003.223. [DOI] [PubMed] [Google Scholar]
- 12.Spravchikov N, Sizyakov G, Gartsbein M, Accili D, Tennenbaum T, Wertheimer E. Glucose effects on skin keratinocytes: Implications for diabetes skin complications. Diabetes. 2001;50:1627–1635. doi: 10.2337/diabetes.50.7.1627. [DOI] [PubMed] [Google Scholar]
- 13.Rosse C, Linch M, Kermorgant S, Cameron AJ, Boeckeler K, Parker PJ. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol. 2010;11:103–112. doi: 10.1038/nrm2847. [DOI] [PubMed] [Google Scholar]
- 14.Ohno S. Intercellular junctions and cellular polarity: The PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr Opin Cell Biol. 2001;13:641–648. doi: 10.1016/S0955-0674(00)00264-7. [DOI] [PubMed] [Google Scholar]
- 15.Yin J, Liu Z, Li H, Sun J, Chang X, Liu J, He S, Li B. Association of PKCζ expression with clinicopathological characteristics of breast cancer. PLoS One. 2014;9:e90811. doi: 10.1371/journal.pone.0090811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler AM, Scotti Buzhardt ML, Li S, Smith KE, Fields AP, Murray NR. Protein kinase C zeta regulates human pancreatic cancer cell transformed growth and invasion through a STAT3-dependent mechanism. PLoS One. 2013;8:e72061. doi: 10.1371/journal.pone.0072061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun R, Gao P, Chen L, Ma D, Wang J, Oppenheim JJ, Zhang N. Protein kinase C zeta is required for epidermal growth factor-induced chemotaxis of human breast cancer cells. Cancer Res. 2005;65:1433–1441. doi: 10.1158/0008-5472.CAN-04-1163. [DOI] [PubMed] [Google Scholar]
- 18.Huang S, Ouyang N, Lin L, Chen L, Wu W, Su F, Yao Y, Yao H. HGF-induced PKCζ activation increases functional CXCR4 expression in human breast cancer cells. PLoS One. 2012;7:e29124. doi: 10.1371/journal.pone.0029124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen R, Wang Y, Liu Y, Zhang Q, Zhang X, Zhang F, Shieh CH, Yang D, Zhang N. Quantitative study of the interactome of PKCζ involved in the EGF-induced tumor cell chemotaxis. J Proteome Res. 2013;12:1478–1486. doi: 10.1021/pr3011292. [DOI] [PubMed] [Google Scholar]
- 20.Yao H, Hwang JW, Moscat J, Diaz-Meco MT, Leitges M, Kishore N, Li X, Rahman I. Protein kinase C zeta mediates cigarette smoke/aldehyde- and lipopolysaccharide-induced lung inflammation and histone modifications. J Biol Chem. 2010;285:5405–5416. doi: 10.1074/jbc.M109.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leverence JT, Medhora M, Konduri GG, Sampath V. Lipopolysaccharide-induced cytokine expression in alveolar epithelial cells: Role of PKCζ-mediated p47 phox phosphorylation. Chem Biol Interact. 2011;189:72–81. doi: 10.1016/j.cbi.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 22.Huang X, Chen LY, Doerner AM, Pan WW, Smith L, Huang S, Papadimos TJ, Pan ZK. An atypical protein kinase C (PKC zeta) plays a critical role in lipopolysaccharide-activated NF-kappa B in human peripheral blood monocytes and macrophages. J Immunol. 2009;182:5810–5815. doi: 10.4049/jimmunol.0804073. [DOI] [PubMed] [Google Scholar]
- 23.Aveleira CA, Lin CM, Abcouwer SF, Ambrósio AF, Antonetti DA. TNF-α signals through PKCζ/NF-κB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes. 2010;59:2872–2882. doi: 10.2337/db09-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goren I, Müller E, Schiefelbein D, Christen U, Pfeilschifter J, Mühl H, Frank S. Systemic anti-TNFalpha treatment restores diabetes-impaired skin repair in ob/ob mice by inactivation of macrophages. J Invest Dermatol. 2007;127:2259–2267. doi: 10.1038/sj.jid.5700842. [DOI] [PubMed] [Google Scholar]
- 25.Nakayama T, Fujisawa R, Yamada H, Horikawa T, Kawasaki H, Hieshima K, Izawa D, Fujiie S, Tezuka T, Yoshie O. Inducible expression of a CC chemokine liver- and activation-regulated chemokine (LARC)/macrophage inflammatory protein (MIP)-3 alpha/CCL20 by epidermal keratinocytes and its role in atopic dermatitis. Int Immunol. 2001;13:95–103. doi: 10.1093/intimm/13.1.95. [DOI] [PubMed] [Google Scholar]
- 26.Sanz MJ, Hartnell A, Chisholm P, Williams C, Davies D, Weg VB, Feldmann M, Bolanowski MA, Lobb RR, Nourshargh S. Tumor necrosis factor alpha-induced eosinophil accumulation in rat skin is dependent on alpha4 integrin/vascular cell adhesion molecule-1 adhesion pathways. Blood. 1997;90:4144–4152. [PubMed] [Google Scholar]
- 27.Tremblay C, Paradis M, Doré M. Expression of E- and P-selectin in tumor necrosis factor-induced dermatitis in dogs. Vet Pathol. 2001;38:261–268. doi: 10.1354/vp.38-3-261. [DOI] [PubMed] [Google Scholar]
- 28.Speiser W, Kapiotis S, Kopp CW, Simonitsch I, Jilma B, Jansen B, Exner M, Chott A. Effect of intradermal tumor necrosis factor-alpha-induced inflammation on coagulation factors in dermal vessel endothelium. An in vivo study of human skin biopsies. Thromb Haemost. 2001;85:362–367. [PubMed] [Google Scholar]
- 29.Movat HZ, Burrowes CE, Cybulsky MI, Dinarello CA. Acute inflammation and a Shwartzman-like reaction induced by interleukin-1 and tumor necrosis factor. Synergistic action of the cytokines in the induction of inflammation and microvascular injury. Am J Pathol. 1987;129:463–476. [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpe RJ, Margolis RJ, Askari M, Amento EP, Granstein RD. Induction of dermal and subcutaneous inflammation by recombinant cachectin/tumor necrosis factor (TNF alpha) in the mouse. J Invest Dermatol. 1988;91:353–357. doi: 10.1111/1523-1747.ep12475754. [DOI] [PubMed] [Google Scholar]
- 31.Moscat J, Diaz-Meco MT, Wooten MW. Of the atypical PKCs, Par-4 and p62: Recent understandings of the biology and pathology of a PB1-dominated complex. Cell Death Differ. 2009;16:1426–1437. doi: 10.1038/cdd.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz-Meco MT, Moscat J. The atypical PKCs in inflammation: NF-κB and beyond. Immunol Rev. 2012;246:154–167. doi: 10.1111/j.1600-065X.2012.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maldonado RF, Sá-Correia I, Valvano MA. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol Rev. 2016;40:480–493. doi: 10.1093/femsre/fuw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harada Y, Edamatsu H, Kataoka T. PLCε cooperates with the NF-κB pathway to augment TNFα-stimulated CCL2/MCP1 expression in human keratinocyte. Biochem Biophys Res Commun. 2011;414:106–111. doi: 10.1016/j.bbrc.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 35.Raingeaud J, Pierre J. Interleukin-4 downregulates TNFalpha-induced IL-8 production in keratinocytes. FEBS Lett. 2005;579:3953–3959. doi: 10.1016/j.febslet.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 36.Youn GS, Kwon DJ, Ju SM, Choi SY, Park J. Curcumin ameliorates TNF-α-induced ICAM-1 expression and subsequent THP-1 adhesiveness via the induction of heme oxygenase-1 in the HaCaT cells. BMB Rep. 2013;46:410–415. doi: 10.5483/BMBRep.2013.46.8.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nigro P, Abe J, Woo CH, Satoh K, McClain C, O' Dell MR, Lee H, Lim JH, Li JD, Heo KS, et al. PKCzeta decreases eNOS protein stability via inhibitory phosphorylation of ERK5. Blood. 2010;116:1971–1979. doi: 10.1182/blood-2010-02-269134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang H, Baudouin C, Behar-Cohen F, Crisanti P, Omri B. Protein kinase C-zeta mediates retinal degeneration in response to TNF. J Neuroimmunol. 2007;183:104–110. doi: 10.1016/j.jneuroim.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Y, Fishelevich R, Petrali JP, Zheng L, Anatolievna MA, Deng A, Eckert RL, Gaspari AA. Activation of keratinocyte protein kinase C zeta in psoriasis plaques. J Invest Dermatol. 2008;128:2190–2197. doi: 10.1038/jid.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaalamo M, Weckroth M, Puolakkainen P, Kere J, Saarinen P, Lauharanta J, Saarialho-Kere UK. Patterns of matrix metal-loproteinase and TIMP-1 expression in chronic and normally healing human cutaneous wounds. Br J Dermatol. 1996;135:52–59. doi: 10.1111/j.1365-2133.1996.tb03607.x. [DOI] [PubMed] [Google Scholar]
- 41.Wong VW, Garg RK, Sorkin M, Rustad KC, Akaishi S, Levi K, Nelson ER, Tran M, Rennert R, Liu W, et al. Loss of keratinocyte focal adhesion kinase stimulates dermal proteolysis through upregulation of MMP9 in wound healing. Ann Surg. 2014;260:1138–1146. doi: 10.1097/SLA.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 42.Reiss MJ, Han YP, Garcia E, Goldberg M, Yu H, Garner WL. Matrix metalloproteinase-9 delays wound healing in a murine wound model. Surgery. 2010;147:295–302. doi: 10.1016/j.surg.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood. 2000;96:2673–2681. [PubMed] [Google Scholar]
- 44.Opdenakker G, Van den Steen PE, Dubois B, Nelissen I, Van Coillie E, Masure S, Proost P, Van Damme J. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol. 2001;69:851–859. [PubMed] [Google Scholar]
- 45.Hussain S, Assender JW, Bond M, Wong LF, Murphy D, Newby AC. Activation of protein kinase Czeta is essential for cytokine-induced metalloproteinase-1, -3, and -9 secretion from rabbit smooth muscle cells and inhibits proliferation. J Biol Chem. 2002;277:27345–27352. doi: 10.1074/jbc.M111890200. [DOI] [PubMed] [Google Scholar]
- 46.Basso FG, Pansani TN, Turrioni AP, Soares DG, de Souza Costa CA, Hebling J. Tumor necrosis factor-α and interleukin (IL)-1β, IL-6, and IL-8 impair in vitro migration and induce apoptosis of gingival fibroblasts and epithelial cells, delaying wound healing. J Periodontol. 2016;87:990–996. doi: 10.1902/jop.2016.150713. [DOI] [PubMed] [Google Scholar]
- 47.Lei M, Bai X, Yang T, Lai X, Qiu W, Yang L, Lian X. Gsdma3 is a new factor needed for TNF-α-mediated apoptosis signal pathway in mouse skin keratinocytes. Histochem Cell Biol. 2012;138:385–396. doi: 10.1007/s00418-012-0960-1. [DOI] [PubMed] [Google Scholar]
- 48.Sun J, Han J, Zhao Y, Zhu Q, Hu J. Curcumin induces apoptosis in tumor necrosis factor-alpha-treated HaCaT cells. Int Immunopharmacol. 2012;13:170–174. doi: 10.1016/j.intimp.2012.03.025. [DOI] [PubMed] [Google Scholar]