Abstract
Mesenchymal stem cells (MSCs) differentiate into multiple lineages and are a promising source of cells for clinical use. Previously, we found that the gene distal-less homeobox 5 (DLX5) is specifically expressed in MSCs with osteogenic potential. Understanding the mechanism of osteogenesis is necessary for successful bone regeneration using MSCs. The aim of this study was to examine the function of the DLX5 gene in MSCs during osteogenesis (bone development). We analyzed the possible association between DLX5 expression and osteogenesis-, chondrogenesis- and adipogenesis-related gene expression in different cells isolated from bone marrow and cord blood. Differentiation capacity was assessed by observing morphological changes, monitoring gene expression patterns, and staining with Von Kossa, safranin O, and Oil Red O. Suppression of DLX5 expression by means of a small interfering RNA (siRNA) downregulated osteogenic markers and reduced the signs of calcium mineralization. Tanshinone IIA is a known small molecule activator of bone morphogenetic protein (BMP) signaling. Here, we report that induction of DLX5 by tanshinone IIA in MSCs enhanced osteogenic differentiation. In addition, we showed that tanshinone IIA (as a mediator of BMP2 signaling) activates runt-related transcription factor 2 (RUNX2) in MSCs and initiates calcium mineralization during osteogenesis. Taken together, these findings indicate that, in MSCs, DLX5 is a master regulator of osteogenesis. Furthermore, tanshinone IIA may be valuable for stem cell-based therapies of certain bone diseases.
Keywords: distal-less homeobox 5, mesenchymal stem cells, osteogenesis, tanshinone IIA
Introduction
Mesenchymal stem cells (MSCs) derived from various sources are valuable in regenerative medicine, including bone repair, because they can differentiate into multiple cell lineages, including osteoblasts, chondrocytes and adipocytes (1,2). MSCs derived from different tissues have similar characteristics, but differ in their molecular profiles and differentiation potential (3).
Recently, MSCs have been applied to bone tissue engineering with a regenerative medicine approach (4). Osteogenic differentiation of MSCs is intricately regulated by multiple transcription factors and various cytokines and hormones (5–7). Previously, we found that distal-less homeobox 5 (DLX5), a homeodomain transcription factor encoded by a mammalian homolog of one of the Drosophila distal-less (DLL/DLX) genes that regulates the development of multiple cell types, is only expressed in MSCs with osteogenic potential (3). The discovery led us to examine whether DLX5 is critically involved in the differentiation of MSCs into osteoblasts.
Homeobox-containing genes play a key role as regulators of skeletal development (8). DLX genes that encode homeobox-containing transcription factors function in several developmental processes, including osteoblast development (9,10). DLX5, which is involved in developing bone, cartilage, and teeth, is a member of the distal-less homeobox domain family (11–14). Overexpression of DLX5 is known to stimulate bone differentiation, and DLX5-null mice exhibit abnormal osteogenesis (15–18). Although numerous studies strongly suggest that DLX5 is involved in osteogenesis, its functional role in this process is still obscure.
Here, we investigated the regulatory role of DLX5 in osteogenic differentiation of bone marrow- and cord blood-derived MSCs by examining the effects of DLX5 inhibition and the expression levels of osteogenesis-associated genes, including bone morphogenetic protein 2 (BMP2) and runt-related transcription factor 2 (RUNX2). BMP2 and RUNX2 play essential roles in bone development and maintenance by collaborating with other signaling molecules; however, they are insufficient to induce osteogenic differentiation (19,20).
The aim of this study was to examine the key regulators of osteogenesis in MSCs. DLX5 is regulated by BMP2, an inducer of osteogenesis (21,22). To investigate the effects of DLX5 on osteogenic differentiation of MSCs, we examined osteogenic factors (DLX5 and RUNX2), chondrogenic factors [BMP7 and sex determining region Y-box 9 (SOX9)], and adipogenic factors [peroxisome proliferator-activated receptor γ (PPARG) and CCAAT-enhancer binding protein α (C/EBPA)]. We demonstrated that the induction of DLX5 led to osteoblast differentiation with the expression of several osteoblast markers, whereas the knockdown of DLX5 expression inhibited the osteogenesis of MSCs. Our data indicate that DLX5 is the master transcription factor stimulating the osteogenic factor RUNX2 through BMP2 signaling during osteogenesis.
Furthermore, we aimed to ascertain whether activation of DLX5 and/or BMP2 signaling by certain chemicals could induce osteogenic differentiation in MSCs. Tanshinone IIA is a major active phytochemical derived from phenanthren-equinone, which can be isolated from the roots of Salvia miltiorrhiza. It was found to enhance BMP2-stimulated differentiation of C2C12 cells into osteoblasts via p38 activation (23). For the first time, we evaluated the effect of tanshinone IIA on the differentiation of MSCs into osteoblasts. This study demonstrated that tanshinone IIA affects osteogenesis from MSCs by augmenting DLX5.
These findings may be important for regenerative medicine, facilitating an increase in MSCs with osteogenic potential. Further, tanshinone IIA, as a small-molecule activator of DLX5 and BMP signaling, could be one of the key molecules in DLX5-induced osteogenesis of MSCs.
Materials and methods
Cells
Bone marrow and umbilical cord blood were collected from healthy donors after obtaining written informed consent. This study was approved by the Institutional Review Boards of Severance Hospital of Yonsei University Health System, Seoul, Korea. As previously described, mononuclear cells were isolated by Ficoll-Hypaque density gradient centrifugation (Pharmacia Biotech, Uppsala, Sweden) and the MSCs were cultured using the plastic adherence method (24). The cells were cultured at 37°C with 5% CO2, and the medium [DMEM-low glucose supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) (all from Invitrogen, Carlsbad, CA, USA)] was changed every 3 or 4 days. Tanshinone IIA from Sigma-Aldrich (St. Louis, MO, USA) was used for this study. During cultivation, cells were photographed under an inverted phase microscope (Olympus IX-71; Olympus, Tokyo, Japan) to compare morphologies.
Differentiation
To cause MSCs to differentiate into osteoblasts, chondrocytes, and adipocytes, bone marrow- and cord blood-derived MSCs were cultured in osteogenic induction medium, chondrogenic induction medium, and adipogenic induction medium for 3 weeks (Cambrex, Lonza, MD, USA). Osteoinductive medium-treated cells were used as the control. The medium was changed every 3 or 4 days, and the cells intended for chondrogenic differentiation were treated with 10 ng/ml transforming growth factor (TGF)-β3 (Cambrex) whenever the medium was replaced. For analysis, the induced cells were stained by Von Kossa to confirm osteogenesis, safranin O to confirm chondrogenesis, and Oil Red O to confirm adipogenesis. Images of the stained cells were captured using a phase microscope (Olympus IX-71; Olympus).
RT-PCR
Total RNA was extracted using TRIzol reagent, and standard reverse transcription (RT) was carried out using transcriptase II (both from Invitrogen). RT-PCR was performed using PCR primers (Bioneer, Daejeon, Korea) and annealing temperatures listed in Table I. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. The signal intensity of the product was normalized to the respective GAPDH signal intensity. Osteoinductive medium-treated cells were used as control.
Table I.
Primer sequences.
| Gene name | Primer sequences (5′-3′) | Annealing temperature (°C) | Product size (bp) |
|---|---|---|---|
| BMP2 | Forward: CGAGGTCCTGAGCGAGTTCGAG | ||
| Reverse: TGGCAGTAAAAGGCGTGATACC | 60 | 838 | |
| RUNX2 | Forward: GACCAGTCTTACCCCTCCTACC | ||
| Reverse: CTGCCTGGCTCTTCTTACTGAG | 58 | 190 | |
| DLX5 | Forward: ACCATCCGTCTCAGGAATCG | ||
| Reverse: ACCTTCTCTGTAATGCGGCC | 60 | 384 | |
| GAPDH | Forward: GTGGTCTCCTCTGACTTCAACA | ||
| Reverse: CTCTTCCTCTTGTGCTCTTGCT | 62 | 210 | |
| BMP7 | Forward: CCAACGTCATCCTGAAGAAATAC | ||
| Reverse: GCTTGTAGGATCTTGTTCATTGG | 60 | 271 | |
| SOX9 | Forward: GCCGGGCAAGGCTGACCTGAAG | ||
| Reverse: TTCTGGTGGTCGGTGTAGTCGT | 62 | 605 | |
| PPARG | Forward: TCTCTCCGTAATGGAAGACC | ||
| Reverse: GCATTATGAGACATCCCCAC | 55 | 474 | |
| C/EBPA | Forward: CCAAGAAGTCGGTGGACAAGAA | ||
| Reverse: TCATTGTCACTGGTCAGCTCCA | 62 | 145 | |
| Osterix | Forward: TAATGGGCTCCTTTCACCTG | ||
| Reverse: CACTGGGCAGACAGTCAGAA | 60 | 161 | |
| Osteopontin | Forward: GAGACCCTTCCAAGTAAGTCCA | ||
| Reverse: GATGTCCTCGTCTGTAGCATCA | 62 | 354 | |
| Type I collagen | Forward: CACAGAGGTTTCAGTGGTTTGG | ||
| Reverse: GCACCAGTAGCACCATCATTTC | 62 | 191 | |
| AP2 | Forward: AAGAAGTAGGAGTGGGCTTTGC | ||
| Reverse: CCACCACCAGTTTATCATCCTC | 62 | 381 |
BMP2, bone morphogenetic protein; RUNX2, runt-related transcription factor 2; DLX5, distal-less homeobox 5; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; SOX9, sex determining region Y-box 9; PPARG, peroxisome proliferator-activated receptor γ; C/EBPA, CCAAT-enhancer binding protein α.
Small interfering RNA (siRNA) gene silencing
Specific knockdown of gene expression was performed using siRNA (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) specific for DLX5. Briefly, 2×105 MSCs were transfected with 10 µM of negative control or targeted siRNA according to the manufacturer's protocol. Following incubation for 7 h at 37°C and 5% CO2, normal growth medium was added. After one day, medium was replaced with fresh normal growth medium. The effect of gene knockdown by siRNA was evaluated by RT-PCR assay. MSCs were treated with DLX5-siRNA when medium was replaced for the entire induction period.
Cell viability test
The viability of chemically treated cells was analyzed by the trypan blue exclusion method (Invitrogen). Briefly, cells were seeded at a density of 5×104 cells in 12-well plates (Nunc, Roskilde, Denmark). The next day, 3, 6, 12, 24 or 48 µM of tanshinone IIA was added to the cells. After 3 days, the cells were harvested and trypan blue-stained cells were counted.
Analysis of calcium concentration
Following osteogenic induction, the calcium content of cells was determined using a Calcium (CPC) LiquiColor Test (Stanbio Laboratory, Boerne, TX, USA) according to the manufacturer's instructions. Briefly, the cells were washed with phosphate-buffered saline (PBS; Invitrogen) and 0.5 N HCl was added to the cells. The cells were harvested and transferred to a new tube. After shaking for 3 h with an orbital shaker, the supernatant was transferred to a new tube for analysis. Color and base reagents were added to the supernatant, and then absorbances were detected at 550 nm. The cells cultured in DMEM were used as the control.
Statistical analysis
Quantitative data are expressed as the means ± standard deviation (SD). Statistical comparisons were performed by a Student's t-test and one-way analysis of variance (ANOVA) with post-hoc Bonferroni corrections. The differences were considered statistically significant at P<0.05.
Results
Characterization of bone marrow- and cord blood-derived MSCs
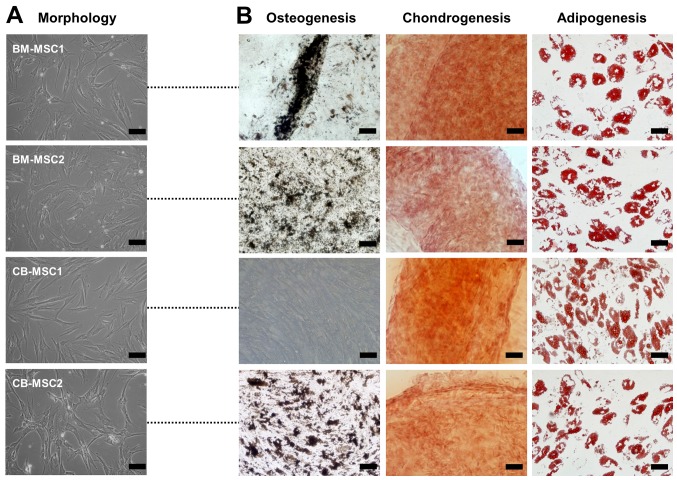
All MSCs derived from bone marrow and cord blood showed a similar spindle-shaped morphology (Fig. 1A). Surface markers of the MSCs were analyzed, and the results showed that all cells exhibited similar immunophenotypic patterns. The cells were positive for CD29, CD44, CD73, CD90 and CD105, all known markers of MSCs, whereas the MSCs were negative for markers of endothelial and hematopoietic cells such as CD14, CD31, CD34, CD45 and CD106 (data not shown). These results confirmed that the cultured cells expressed typical MSC surface markers. To determine their differentiation capacity, the cells were induced to display osteogenic, chondrogenic, or adipogenic phenotypes. Of the MSCs derived from bone marrow and cord blood, one sample of cord blood MSCs (CB-MSC1) did not differentiate into osteoblasts despite a sufficient induction period, whereas the other MSCs exhibited tri-lineage differentiation potential, developing into osteoblasts, chondrocytes and adipocytes (Fig. 1B). Together, these data indicate that not all MSCs with fibroblast-like morphologies and MSC surface proteins have tri-lineage differentiation capacities.
Figure 1.
Morphology and differentiation potential of mesenchymal stem cells (MSCs). (A) Phase contrast images of MSCs derived from bone marrow and cord blood (scale bar, 100 µm; magnification, ×200). (B) Differentiation capacity was examined after induction. Osteogenesis was verified by Von Kossa staining (scale bar, 100 µm; magnification, ×200). Chondrogenesis was investigated by safranin O staining (scale bar, 100 µm; magnification, ×200). Adipogenesis was examined by Oil Red O staining (scale bar, 50 µm; magnification, ×400).
Osteogenesis and DLX5 expression of MSCs
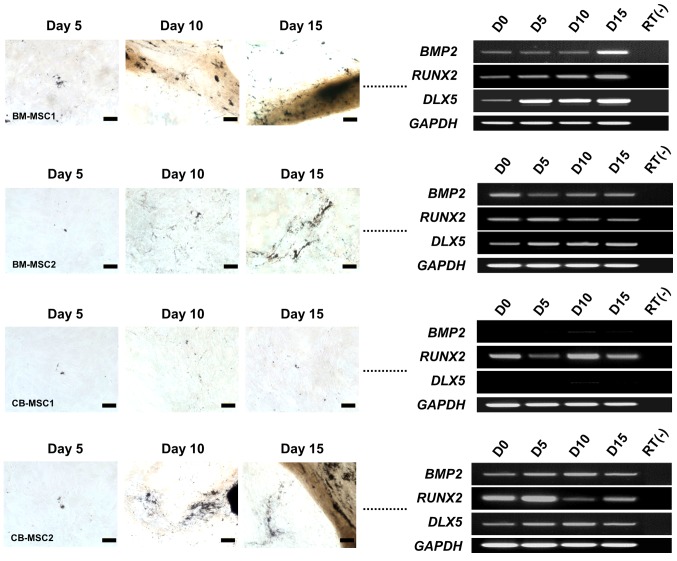
To investigate osteogenic molecular profiles associated with morphological changes, we performed an RT-PCR analysis of specific osteogenesis markers, namely BMP2, RUNX2 and DLX5, during the induction of bone marrow- and cord blood-derived MSCs from different donors. BMP2, RUNX2 and DLX5 were expressed in all MSCs that differentiated into osteoblasts, regardless of the induction period (Fig. 2). However, CB-MSC1, which did not differentiate into an osteogenic phenotype, did not express BMP2 and DLX5 at any time in the induction environment. Interestingly, RUNX2 was independently expressed in all MSCs, regardless of their osteogenic potential (Fig. 2). These results coincide with previous data, confirming DLX5 as a marker for the osteogenic potential of MSCs (3). Based on these results, we noted that DLX5 with BMP2 signaling may be the only critical factors for osteogenesis of MSCs.
Figure 2.
Time course images of osteogenic differentiation and expression of osteogenesis-related markers. Top panels: images of BM-MSC1 induced by osteogenic medium, and RT-PCR analysis of osteogenesis-associated markers in BM-MSC1. Second row: images of BM-MSC2 differentiated by osteogenic medium, and RT-PCR analysis of osteogenesis-related markers in BM-MSC2. Third row: images of CB-MSC1 induced by osteogenic medium, and RT-PCR analysis of osteogenesis-associated markers in CB-MSC1. Lower panels: images of CB-MSC2 induced by osteogenic medium, and RT-PCR analysis of osteogenesis-related markers in CB-MSC2. Osteogenesis was evaluated by Von Kossa staining (scale bar, 100 µm; magnification, ×200).
Effect of DLX5 knockdown on the differentiation potential of MSCs
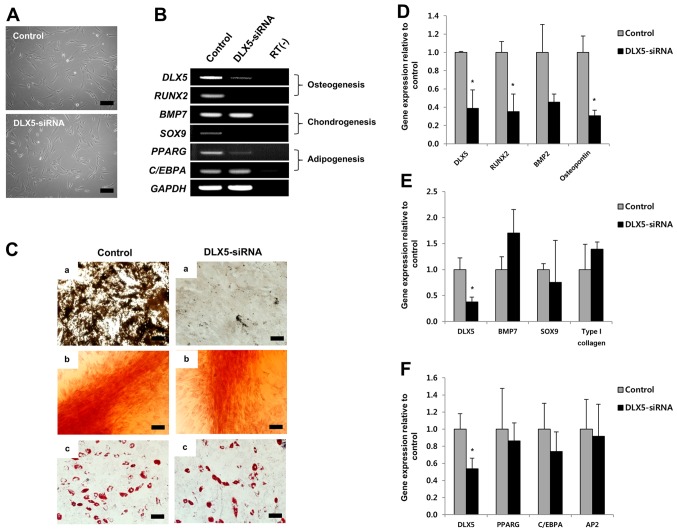
To examine the role of DLX5 in the tri-lineage differentiation of MSCs, we employed siRNA-mediated knockdown of DLX5, using DLX5-expressing cells. The morphologies of cultured MSCs before induction were unaffected, compared to those of the control, by short-term treatment with DLX5-siRNA (Fig. 3A). RT-PCR results showed that DLX5-siRNA substantially decreased expression of the DLX5 gene and completely silenced the osteogenic marker gene RUNX2 and chondrogenic marker gene SOX9. C/EBPA of the adipogenic marker genes was unaffected by DLX5-siRNA treatment, while PPARG expression was slightly decreased (Fig. 3B). These results indicate that osteogenesis of MSCs can be markedly affected by DLX5-siRNA knockdown.
Figure 3.
Changes in mesenchymal stem cells (MSCs) following knockdown of distal-less homeobox 5 (DLX5). (A) Phase contrast images before and after silencing of DLX5 (scale bar, 200 µm; magnification, ×100). (B) RT-PCR analysis of osteogenesis-, adipogenesis- and chondrogenesis-associated genes in the control and DLX5 siRNA-transfected MSCs. Decreased osteogenic differentiation of MSCs following knockdown of DLX5. (C) Inhibition of DLX5 expression prevents osteogenesis of MSCs. The differentiation capacity of MSCs was analyzed after induction. (a) Osteogenesis assessed by Von Kossa staining. (b) Chondrogenesis assessed by safranin O staining. (c) Adipogenesis assessed by Oil Red O staining (scale bar, 100 µm). Relative mRNA expression levels of differentiation-associated markers in the control and DLX5-siRNA-transfected MSCs after (D) osteogenic induction, (E) chondrogenic induction, and (F) adipogenic induction. *P<0.05.
We next performed a differentiation assay in the presence of DLX5-siRNA. Surprisingly, the osteogenic capacity of MSCs treated with DLX5-siRNA was significantly decreased, whereas chondrogenic and adipogenic capacities were similar, relative to that of the control, although MSCs did not express the SOX9 gene following DLX5-siRNA treatment (Fig. 3C). We then analyzed gene expression levels related to tri-lineage differentiation by RT-PCR after induction. Expression of the following genes was evaluated: DLX5, RUNX2, BMP2 and osteopontin for osteogenesis; DLX5, BMP7, SOX9 and type I collagen for chondrogenesis; and DLX5, PPARG, C/EBPA and AP2 for adipogenesis. The levels of RUNX2 and osteopontin gene expression were significantly decreased relative to the control by inhibition of DLX5 (Fig. 3D), whereas no significant differences were detected in the expression of chondrogenesis- (Fig. 3E) or adipogenesis- (Fig. 3F) related genes. Relative gene expression was normalized to that of GAPDH, the internal control. These results strongly suggest that DLX5 is the most powerful and specific transcription factor for osteogenic differentiation.
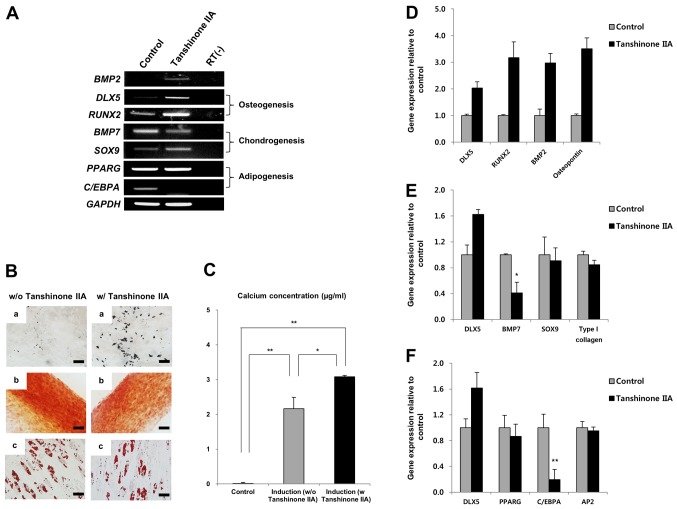
Tanshinone IIA induces DLX5 through BMP2 signaling in MSCs
Tanshinone IIA, a major active phytochemical, is involved in bone metabolism. It has a wide range of biological activities, including anti-inflammation and anti-oxidation (25-27). Moreover, tanshinone IIA is known to enhance BMP-2 stimulation of cells to differentiate into osteoblasts (23). Ultimately, stimulation by tanshinone IIA induces osteogenesis via regulation of osteogenic factors, including BMP2 and DLX5.
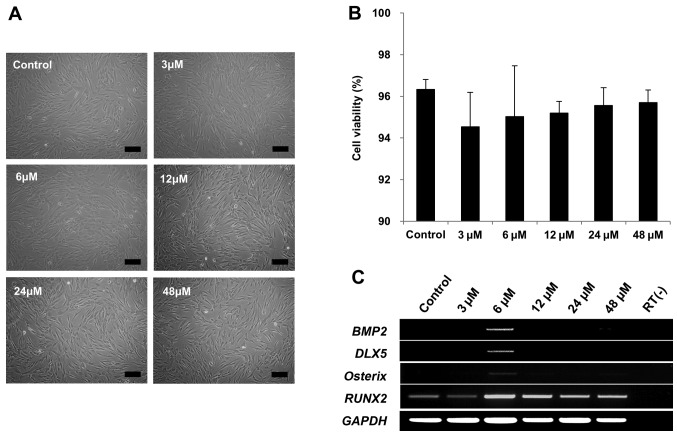
Therefore, we tested whether tanshinone IIA could induce DLX5 in DLX5 not-expressing MSCs. Cell morphologies were not affected by 3, 6, 12, 24 or 48 µM of tanshinone IIA treatment (Fig. 4A). In addition, cell viability of >90% was maintained with tanshinone IIA treatment (Fig. 4B). Remarkably, BMP2, DLX5 and osterix genes were only induced in response to 6 µM of tanshinone IIA treatment, as early as after 3 days of cultivation, indicating the activation of DLX5 by the BMP2 pathway. However, RUNX2 was similarly expressed (Fig. 4C). Taken together, these results show that tanshinone IIA can induce DLX5, as well as the most prominent factors of osteogenesis.
Figure 4.
Effect of tanshinone IIA on osteogenesis. (A) Mesenchymal stem cells (MSCs) treated with tanshinone IIA were photographed after 3 days (scale bar, 200 µm; magnification, ×100). (B) Cell viability was determined by trypan blue staining. The cell viability of the tanshinone IIA-treated MSCs was >90%. (C) Induction of osteogenesis-associated genes by tanshinone IIA treatment. Treatment only with 6 µM tanshinone IIA induced BMP2, distal-less homeobox 5 (DLX5) and osterix genes.
Tanshinone IIA enhances osteogenesis of MSCs by inducing DLX5 with BMP2
We next treated DLX5 not-expressing MSCs with 6 µM tanshinone IIA to induce osteogenesis. After tanshinone IIA treatment, we did not find any differences in our morphological investigation. Subsequently, the effect of tanshinone IIA on the osteogenic potential of MSCs was evaluated by analyzing the expression levels of genes associated with osteogenic differentiation and by visualizing the staining of induced cells. Tanshinone IIA significantly induced BMP2 and DLX5, as well as upregulated RUNX2 genes involved in osteogenesis. The expression of SOX9, which is involved in chondrogenesis, was similarly upregulated, compared to that of the control, despite tanshinone IIA treatment, whereas BMP7 expression was decreased (Fig. 5A). Interestingly, expression of C/EBPA, a transcription factor for adipogenesis, was completely inhibited by tanshinone IIA (Fig. 5A). The differentiation assay revealed that tanshinone IIA specifically enhanced osteogenesis of MSCs (Fig. 5B). This finding was confirmed by calcium deposition assay, as shown in Fig. 5C. Furthermore, tanshinone IIA-mediated enhancement of DLX5 through the induction of BMP2 upregulated mRNA expression of RUNX2 and osteopontin during osteogenic differentiation of MSCs (Fig. 5D). As shown in Fig. 5A, we again confirmed that the expression of BMP7 was decreased significantly during chondrogenesis (Fig. 5E), and that C/EBPA expression was suppressed by tanshinone IIA during adipogenic differentiation (Fig. 5F). Relative gene expression in the differentiated MSCs was normalized to GAPDH, the internal control. Surprisingly, tanshinone IIA-treated MSCs differentiated into chondrocytes and adipocytes despite the suppression of BMP7 and C/EBPA genes (Fig. 5B). Taken together, these results indicate that tanshinone IIA induces osteogenesis in DLX5 not-expressing MSCs by activating DLX5 through BMP2 expression.
Figure 5.
Changes in mesenchymal stem cells (MSCs) following tanshinone IIA treatment. (A) RT-PCR analysis of osteogenesis-, chondrogenesis- and adipogenesis-associated genes in the control and tanshinone IIA-induced MSCs. Increased osteogenic differentiation of MSCs by tanshinone IIA. (B) Induction of distal-less homeobox 5 (DLX5) expression enhanced osteogenesis of DLX5 not-expressing MSCs. The differentiation capacity of the cells was analyzed after induction. (a) Osteogenesis assessed by Von Kossa staining (scale bar, 50 µm). (b) Chondrogenesis assessed by safranin O staining (scale bar, 100 µm). (c) Adipogenesis assessed by Oil Red O staining (scale bar, 50 µm). (C) Calcium concentrations were measured in triplicate using a Calcium LiquiColor test. Relative mRNA expression levels of differentiation-associated markers in the control and DLX5-induced MSCs after (D) osteogenic induction, (E) chondrogenic induction, and (F) adipogenic induction. *P<0.05 and **P<0.01.
Discussion
MSCs derived from various tissues have become a preferred cell type in the field of regenerative medicine due to their plastic and immunosuppressive properties (28). Although stem cells hold great promise for future therapeutic applications, clinical applications using these cells have been stymied by an insufficient understanding of stem cell biology, including the complex genetic processes in these cells. Therefore, further characterization of stem cells via diverse approaches such as genomics and proteomics will be critical for a better understanding and utilization of stem cells.
Previous research on MSCs from different sources has documented their variable differentiation potential and has shown that this variation in osteogenic potential depends on DLX5 gene expression (3). Consistent with the results of a previous study, DLX5 expression was not detected in MSCs that did not have the capacity to differentiate into osteoblasts. In addition, BMP2 gene expression was not observed in this study when DLX5 not-expressing MSCs were maintained in an osteogenic environment. Differentiation of stem cells is a complex process governed by various genetic networks, and the biological functions of genes associated with MSC differentiation remain unclear. In the present study, we aimed to investigate the precise role of the DLX5 gene during the osteogenesis of MSCs, including whether the DLX5 gene is important for initiating osteogenesis and whether the gene is sufficient to completely drive osteogenesis from MSCs.
DLX5, a member of the DLX family of homeobox genes, is known to be a key regulator of differentiation involved in developing skeletal elements and of osteogenesis and chondrogenesis in the formation of hard tissues (29). Several studies suggest that DLX5 acts as a modulator of osteogenesis in various cell types (18,30). However, the mechanism underlying osteogenic differentiation, including the role of DLX5, is still controversial, especially in MSCs. In this study, we used MSCs derived from bone marrow and cord blood, less than 5 passages, and expressing and/or not expressing the DLX5 gene to identify the effects, including the effects on genes activated and inactivated by DLX5 in the course of differentiation. In order to investigate the role of DLX5, we profiled morphological and gene expression changes associated with osteogenesis of MSCs. As mentioned above, DLX5 not-expressing MSCs without BMP2 expression failed to differentiate into osteoblasts. However, RUNX2 was consistently expressed during osteogenic induction, irrespective of the expression of BMP2 and DLX5. To further examine the effect of DLX5 on osteogenesis, siRNA, which targets the DLX5 gene, was used to inhibit endogenous DLX5 expression in MSCs. Knockdown of DLX5 using siRNA did not alter the morphology and the proliferation rate of the cells. Seventy-two hours after siRNA transfection, RUNX2 and SOX9 genes specific for osteogenesis and chondrogenesis, respectively, were inhibited in the cultured cells. In addition, osteogenic differentiation of MSCs was significantly suppressed by DLX5-siRNA, with a decrease in osteopontin gene expression compared to the control. In contrast, the chondrogenic and adipogenic potential of these cells was unaffected by DLX5-siRNA, as proteoglycans for chondrogenesis and neutral lipids for adipogenesis were similarly detected by immunohistochemical staining in control cells exposed to inductive conditions. These results indicate that DLX5 drives the osteogenic differentiation program in MSCs.
Tanshinone IIA is a major active phytochemical that is isolated from the roots of S. miltiorrhiza and enhances BMP2-stimulated differentiation of myoblasts into osteoblasts (23). However, there is little research on the effects of tanshinone IIA on the osteogenic differentiation of MSCs. To examine the effects of tanshinone IIA on MSCs, DLX5 not-expressing cells were cultured with tanshinone IIA. Our data showed that DLX5 induced by tanshinone IIA activated osteogenic marker genes, including osterix, RUNX2 and osteopontin, in cooperation with BMP2, with cell morphologies that remained similar to control cells, whereas tanshinone IIA suppressed BMP7 gene (chondrogenesis) and C/EBPA gene (adipogenesis) expression. These results are in line with previous studies showing that DLX5 plays a role in BMP2-induced osteogenesis through upregulation of the RUNX2 gene, and that it functions as part of the BMP signaling pathway (21,31). In addition, these results suggest that tanshinone IIA is involved in the BMP2 signaling pathway and DLX5-induced osteogenic differentiation. Functional validation with tanshinone IIA was carried out by differentiation assays and PCR analysis. MSCs with strongly upregulated DLX5, RUNX2, BMP2 and osteopontin genes following tanshinone IIA treatment differentiated into osteoblasts and showed significantly increased calcium deposition compared to DLX5 not-expressing cells. However, a higher concentration of tanshinone IIA (6 µM) decreased the osteogenic capacity of MSCs, indicating that osteogenic differentiation following DLX5 induction in treated cells is tanshinone IIA concentration-dependent (data not shown). Additionally, MSCs treated with tanshinone IIA differentiated into chondrocytes and adipocytes despite inhibition of BMP7 and C/EBPA, indicating that these genes may not be essential factors for differentiation. Furthermore, DLX5 may play a role as an osteogenesis determinant through the upregulation of RUNX2 and the downregulation of BMP7 and C/EBPA.
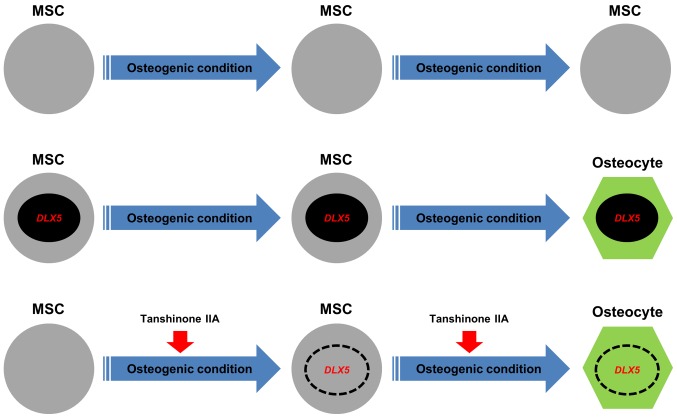
Here, we showed that DLX5 is a specific target of BMP2-induced osteogenesis of MSCs, demonstrating that DLX5 and BMP2 can contribute to RUNX2-independent regulation of osteogenesis. This indicates that RUNX2 induction is not mediated by BMP2 and DLX5 in MSCs as previously reported (32). Additionally, we confirmed that RUNX2 is not essential for the induction of an osteogenic lineage of MSCs, indicating that RUNX2 may function in concert with DLX5 to induce osteogenic differentiation by regulating the expression of osteogenesis-specific markers such as osteopontin. These findings are in agreement with previous results showing that DLX5 plays an important role in the activation of osteogenesis by regulating BMP-induced RUNX2 (22). Moreover, we showed that tanshinone IIA is capable of stimulating DLX5 expression with BMP2, resulting in osteogenic differentiation of MSCs. To the best of our knowledge, we showed for the first time that tanshinone IIA can be used in place of DLX5 to induce differentiate of MSCs into osteoblasts. Fig. 6 shows a schematic model that summarizes the osteogenesis of MSCs by the induction of the DLX5 gene using tanshinone IIA. Our findings contribute to the development of effective bone regeneration therapies for the treatment of bone diseases. Furthermore, tanshinone IIA is a chemical compound that may be used for the treatment of bone diseases; however, our in vitro results require in vivo validation. Additional investigations are required for a deeper understanding of the upstream and downstream signaling pathways related to other osteogenesis-related factors.
Figure 6.
Model summarizing osteogenesis in mesenchymal stem cells (MSCs). Distal-less homeobox 5 (DLX5) and DLX5 inducer, tanshinone IIA, promote the differentiation of MSCs into osteoblasts by upregulating osteogenesis-related genes.
In conclusion, our data showed that DLX5 plays a role as a master transcription factor in osteogenic differentiation, and that tanshinone IIA, coincident with the induction of BMP2, synergistically induces osteogenesis by targeting DLX5.
Acknowledgments
The present study was supported by Grant HI15C0942 from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea.
References
- 1.Koç ON, Lazarus HM. Mesenchymal stem cells: Heading into the clinic. Bone Marrow Transplant. 2001;27:235–239. doi: 10.1038/sj.bmt.1702791. [DOI] [PubMed] [Google Scholar]
- 2.Prockop DJ, Gregory CA, Spees JL. One strategy for cell and gene therapy: Harnessing the power of adult stem cells to repair tissues. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11917–11923. doi: 10.1073/pnas.1834138100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heo JS, Choi Y, Kim HS, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med. 2016;37:115–125. doi: 10.3892/ijmm.2015.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. Cell-based bone tissue engineering. PLoS Med. 2007;4:e9. doi: 10.1371/journal.pmed.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komori T. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem. 2006;99:1233–1239. doi: 10.1002/jcb.20958. [DOI] [PubMed] [Google Scholar]
- 6.Wagner ER, Luther G, Zhu G, Luo Q, Shi Q, Kim SH, Gao JL, Huang E, Gao Y, Yang K, et al. Defective osteogenic differentiation in the development of osteosarcoma. Sarcoma. 2011;2011:325238. doi: 10.1155/2011/325238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev. 2000;21:393–411. doi: 10.1210/edrv.21.4.0403. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Li X, Wang W, Lufkin T. Dlx5 and Dlx6: An evolutionary conserved pair of murine homeobox genes expressed in the embryonic skeleton. Ann NY Acad Sci. 1996;785:38–47. doi: 10.1111/j.1749-6632.1996.tb56242.x. [DOI] [PubMed] [Google Scholar]
- 9.Depew MJ, Lufkin T, Rubenstein JL. Specification of jaw subdivisions by Dlx genes. Science. 2002;298:381–385. doi: 10.1126/science.1075703. [DOI] [PubMed] [Google Scholar]
- 10.Robledo RF, Rajan L, Li X, Lufkin T. The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes Dev. 2002;16:1089–1101. doi: 10.1101/gad.988402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari D, Sumoy L, Gannon J, Sun H, Brown AM, Upholt WB, Kosher RA. The expression pattern of the Distal-less homeobox-containing gene Dlx-5 in the developing chick limb bud suggests its involvement in apical ectodermal ridge activity, pattern formation, and cartilage differentiation. Mech Dev. 1995;52:257–264. doi: 10.1016/0925-4773(95)98113-O. [DOI] [PubMed] [Google Scholar]
- 12.Newberry EP, Latifi T, Towler DA. Reciprocal regulation of osteocalcin transcription by the homeodomain proteins Msx2 and Dlx5. Biochemistry. 1998;37:16360–16368. doi: 10.1021/bi981878u. [DOI] [PubMed] [Google Scholar]
- 13.Ryoo HM, Hoffmann HM, Beumer T, Frenkel B, Towler DA, Stein GS, Stein JL, van Wijnen AJ, Lian JB. Stage-specific expression of Dlx-5 during osteoblast differentiation: Involvement in regulation of osteocalcin gene expression. Mol Endocrinol. 1997;11:1681–1694. doi: 10.1210/mend.11.11.0011. [DOI] [PubMed] [Google Scholar]
- 14.Weiss KM, Ruddle FH, Bollekens J. Dlx and other homeobox genes in the morphological development of the dentition. Connect Tissue Res. 1995;32:35–40. doi: 10.3109/03008209509013703. [DOI] [PubMed] [Google Scholar]
- 15.Acampora D, Merlo GR, Paleari L, Zerega B, Postiglione MP, Mantero S, Bober E, Barbieri O, Simeone A, Levi G. Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development. 1999;126:3795–3809. doi: 10.1242/dev.126.17.3795. [DOI] [PubMed] [Google Scholar]
- 16.Depew MJ, Liu JK, Long JE, Presley R, Meneses JJ, Pedersen RA, Rubenstein JL. Dlx5 regulates regional development of the branchial arches and sensory capsules. Development. 1999;126:3831–3846. doi: 10.1242/dev.126.17.3831. [DOI] [PubMed] [Google Scholar]
- 17.Miyama K, Yamada G, Yamamoto TS, Takagi C, Miyado K, Sakai M, Ueno N, Shibuya H. A BMP-inducible gene, dlx5, regulates osteoblast differentiation and mesoderm induction. Dev Biol. 1999;208:123–133. doi: 10.1006/dbio.1998.9197. [DOI] [PubMed] [Google Scholar]
- 18.Tadic T, Dodig M, Erceg I, Marijanovic I, Mina M, Kalajzic Z, Velonis D, Kronenberg MS, Kosher RA, Ferrari D, et al. Overexpression of Dlx5 in chicken calvarial cells accelerates osteoblastic differentiation. J Bone Miner Res. 2002;17:1008–1014. doi: 10.1359/jbmr.2002.17.6.1008. [DOI] [PubMed] [Google Scholar]
- 19.Maeno T, Moriishi T, Yoshida CA, Komori H, Kanatani N, Izumi S, Takaoka K, Komori T. Early onset of Runx2 expression caused craniosynostosis, ectopic bone formation, and limb defects. Bone. 2011;49:673–682. doi: 10.1016/j.bone.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Rosen V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 2009;20:475–480. doi: 10.1016/j.cytogfr.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Holleville N, Quilhac A, Bontoux M, Monsoro-Burq AH. BMP signals regulate Dlx5 during early avian skull development. Dev Biol. 2003;257:177–189. doi: 10.1016/S0012-1606(03)00059-9. [DOI] [PubMed] [Google Scholar]
- 22.Lee MH, Kim YJ, Kim HJ, Park HD, Kang AR, Kyung HM, Sung JH, Wozney JM, Kim HJ, Ryoo HM. BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-beta 1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J Biol Chem. 2003;278:34387–34394. doi: 10.1074/jbc.M211386200. [DOI] [PubMed] [Google Scholar]
- 23.Kim HJ, Kim SH. Tanshinone IIA enhances BMP-2-stimulated commitment of C2C12 cells into osteoblasts via p38 activation. Amino Acids. 2010;39:1217–1226. doi: 10.1007/s00726-010-0557-8. [DOI] [PubMed] [Google Scholar]
- 24.Sohn HS, Heo JS, Kim HS, Choi Y, Kim HO. Duration of in vitro storage affects the key stem cell features of human bone marrow-derived mesenchymal stromal cells for clinical transplantation. Cytotherapy. 2013;15:460–466. doi: 10.1016/j.jcyt.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Jang SI, Jeong SI, Kim KJ, Kim HJ, Yu HH, Park R, Kim HM, You YO. Tanshinone IIA from Salvia miltiorrhiza inhibits inducible nitric oxide synthase expression and production of TNF-alpha, IL-1beta and IL-6 in activated RAW 264.7 cells. Planta Med. 2003;69:1057–1059. doi: 10.1055/s-2003-45157. [DOI] [PubMed] [Google Scholar]
- 26.Lee SY, Choi DY, Woo ER. Inhibition of osteoclast differentiation by tanshinones from the root of Salvia miltiorrhiza Bunge. Arch Pharm Res. 2005;28:909–913. doi: 10.1007/BF02973876. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Wei Y, Yuan S, Liu G, Lu Y, Zhang J, Wang W. Potential anticancer activity of tanshinone IIA against human breast cancer. Int J Cancer. 2005;116:799–807. doi: 10.1002/ijc.20880. [DOI] [PubMed] [Google Scholar]
- 28.Ménard C, Tarte K. Immunoregulatory properties of clinical grade mesenchymal stromal cells: Evidence, uncertainties, and clinical application. Stem Cell Res Ther. 2013;4:64. doi: 10.1186/scrt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simeone A, Acampora D, Pannese M, D'Esposito M, Stornaiuolo A, Gulisano M, Mallamaci A, Kastury K, Druck T, Huebner K. Cloning and characterization of two members of the vertebrate Dlx gene family. Proc Natl Acad Sci USA. 1994;91:2250–2254. doi: 10.1073/pnas.91.6.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erceg I, Tadić T, Kronenberg MS, Marijanović I, Lichtler AC. Dlx5 regulation of mouse osteoblast differentiation mediated by avian retrovirus vector. Croat Med J. 2003;44:407–411. [PubMed] [Google Scholar]
- 31.Lee MH, Kim YJ, Yoon WJ, Kim JI, Kim BG, Hwang YS, Wozney JM, Chi XZ, Bae SC, Choi KY, et al. Dlx5 specifically regulates Runx2 type II expression by binding to homeodomain-response elements in the Runx2 distal promoter. J Biol Chem. 2005;280:35579–35587. doi: 10.1074/jbc.M502267200. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Song Y, Zhang X, Tang J, Chen J, Chen Y. Msx1/Bmp4 genetic pathway regulates mammalian alveolar bone formation via induction of Dlx5 and Cbfa1. Mech Dev. 2003;120:1469–1479. doi: 10.1016/j.mod.2003.09.002. [DOI] [PubMed] [Google Scholar]