Figure 5. 225Ac-IA-TLs cause significant enhancement of blood-tumor barrier permeability.
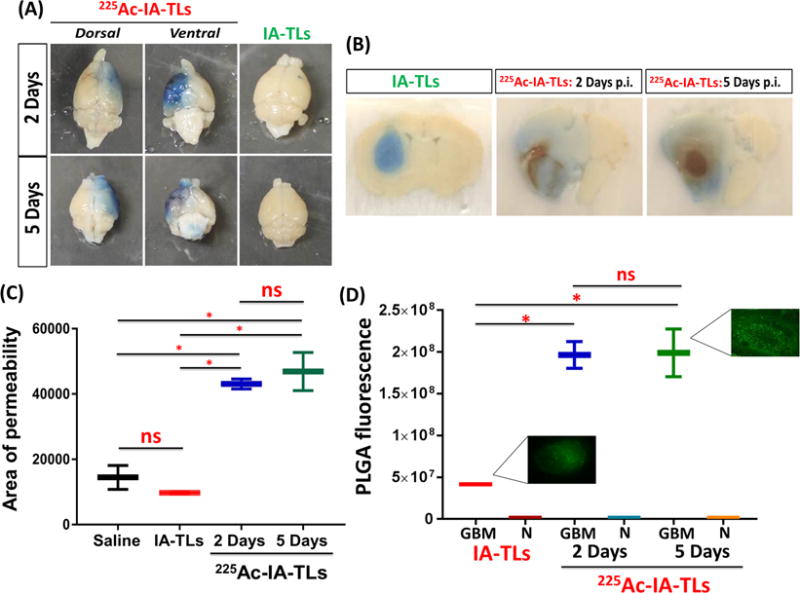
(A) Extravasation of intravenously injected Evans blue dye showed clear enhancement of blood-tumor barrier permeability in nude mice bearing orthotopic glioblastomas that were intracranially infused with 225Ac-IA-TLs 2 days (n=3) and 5 days (n=3) post infusion when compared to mice bearing orthotopic glioblastomas that were intracranially infused with unlabeled IA-TLs. (B) Brain sections showed extensive enhancement of BTB indicated by the extravasation of Evans blue dye in mice intracranially infused with 225Ac-IA-TLs for a period of 5 days when compared to mice intracranially infused with unlabeled IA-TLs. (C) BTB permeability within untreated GBMs, measured using Evans Blue dye permeabilization was found to be similar in mice intracranially infused with saline and unlabeled IA-TLs. Area of extravasation of Evans Blue dye was significantly greater in brains of mice treated with 225Ac-IA-TLs than in untreated mice. (D) Quantification of systemically injected fluorescent PLGA nanoparticles showed that α- particle enhanced BTB could potentially be exploited to deliver diagnostic and therapeutic agents (n=3/group). PLGA fluorescence in and around GBM tissue was measured. GBM= GBM tissue; N= Normal brain region surrounding GBM tissue. Data represented as mean +/− SEM. Student’s t-test and One-way ANOVA was performed to assess differences between experimental groups (*p< 0.001(significant)).
