Graphical abstract

1. Introduction
Loss of the blood-retinal barrier (BRB) contributes to the pathophysiology of a number of retinal diseases, including diabetic retinopathy and neovascular age-related macular degeneration (Frey and Antonetti, 2011; Runkle and Antonetti, 2011). Structurally, the BRB consists of two distinct barriers, the outer BRB consisting of retinal pigment epithelium that regulates transport between the choriocapillaris and the retina, and the inner BRB, which regulates transport across retinal capillaries (Antonetti D.A, 2008; Frey and Antonetti, 2011). The inner BRB relies on tight junction (TJ) proteins between endothelial cells to regulate the flux of ions, molecules and fluid between blood and retinal tissue. TJ disruption leads to vascular permeability, considered a hallmark of retinal vascular diseases including diabetic retinopathy, retinopathy of prematurity and central retinal vein occlusions, among others. Molecular components of tight junctions in vascular endothelial cells include the transmembrane proteins claudins -1, -5, and -12, occludin and tricellulin, junctional adhesion molecules, and endothelial cell selective adhesion molecule, as well as the junctional organizing proteins, zona occludens (ZO-1 and -2), which connect the transmembrane proteins with the cytoskeleton. In addition to TJ sites, adherens junctions (AJ) also regulate paracellular flux, mainly through the expression and regulation of vascular endothelial cadherin (VE-Cadherin) and platelet endothelial cell adhesion molecule (PECAM) (For review see (Dejana, 2004)).
The blood vessels of the retina, like the brain, are connected to a high number of pericytes that envelop the retinal endothelial cells, as well as astrocytes whose end-feet processes wrap around capillaries. Collectively, signals from both pericytes and glial cells are required for formation and maintenance of the BRB properties (Daneman et al., 2010; Runkle and Antonetti, 2011; Shen et al., 2012). Understanding the molecular mechanisms that promote BRB properties in endothelial cell cultures separately from pericytes and glia may provide insight into the development of new therapies for retinal vascular permeability.
Activation of the sonic hedgehog (Shh) signaling pathway is important in a number of developmental processes including neuronal differentiation, axon guidance, and angiogenesis (Fuccillo et al., 2006; Nagase et al., 2008). In the absence of Shh, the Patched-1 (PTCH1) receptor interacts with Smoothened (SMO) receptor inhibiting signal transduction. Upon Shh interaction with PTCH1, SMO activation induces nuclear translocation of Gli transcription factors (Traiffort et al., 2010). However, some recent studies suggest the existence of an additional Shh pathway, independent of Gli activity (Jenkins, 2009). In the brain Shh is secreted from astrocytes to promote vascular barrier properties, by the upregulation of TJ proteins (Alvarez et al., 2011); however, whether Shh promotes stability of the BRB has not been studied yet, and its ability to reverse vascular endothelial growth factor (VEGF) induced permeability is not known.
In this study, we tested the hypothesis that Shh signaling is required for endothelial cell barrier maintenance in a primary culture of retinal endothelial cells. Our data suggest a role for cell autonomous signaling of the Shh/SMO pathway through Gli transcription factors, in tight junction maintenance in endothelial cells. Shh pathway inhibition appears to act in parallel with VEGF to disrupt barrier integrity.
2. Material and Methods
2.1. Primary bovine retinal endothelial cell culture
Primary bovine retinal endothelial cells (BRECs), a well-characterized primary cell preparation used for in vitro studies of retinal vascular permeability, were derived as previously described (Antonetti and Wolpert, 2003; Aveleira et al., 2010). Cells were thawed at passage 2 and grown as previously described (Aveleira et al., 2010). Cells from passage 2-8 were used for all experiments. Briefly, ECIS 8-well chamber slides, transwell inserts, 60mm plates or plastic coverslips, were coated with fibronectin (1 μg/cm2, Sigma, St. Louis, MO) for one hour at room temperature. Cells were plated in MCDB-131 complete media (Life Technologies, Grand Island, NY) with 10% FBS and supplemented with EGF (10 ng/ml, Sigma), Endogro (0.1 mg/ml, Vec Technologies, Rensselaer, NY), and heparin (0.045 mg/ml, Fisher Scientific, Waltham, MA) as previously described (Aveleira et al., 2010). BREC were grown for two days to confluence at 37 degrees Celsius and then the media was changed to MCDB-131 medium supplemented with 1% FBS, 0.01 ml/ml antibiotic/antimycotic (Life Technologies) and hydrocortisone (100 nM, Sigma) for two additional days. At this point VEGF (50 ng/ml), human recombinant Shh (hrShh) (10, 30, 100 and 300 ng/ml; all from R&D systems, Minneapolis, MN), KAAD-Cyclopamine (10 μM, Stemgent, San Diego, CA), Purmorphamine (10 and 100 ng/ml; Tocris Bioscience, Bistrol, UK) or Vismodegib (GDC-0049) (200mg/ml, a gift from Dr. Sunny Wong) were added at the top of the monolayers.
2.2. Trans-endothelial electrical resistance (TEER) measurements
TEER measurements over time were made using the electrical cell-substrate impedance sensing (ECIS) Z-theta system (Applied Biophysics, Troy, NY). For continuous real-time TEER experiments, 45,000 BREC were plated in 8-well chamber slides equipped with gold plated electrode arrays (Applied Biophysics) and monolayer TEER was measured at 4000 Hz once every hour (Tiruppathi et al., 1992). TEER measurements are expressed in total ohms per well within 0.8 cm2 growth area.
2.3. Permeability assay
BREC were plated on 12-mm diameter, 0.4-μm pore size, polyester transwell filters (Costar) at 1×105 cells/well. After the indicated treatments, 10 μM of 70 kDa rhodamine isothiocyanate (RITC) dextran (Sigma) was added to the apical chamber. To determine monolayer permeability, 50 μl aliquots were collected from the basolateral chamber every 30 minutes and after 3.5 hours, 10 μL from the apical chamber was collected and diluted 1:5. Aliquots were quantified using FluorImager 595 (Molecular Dynamics), and diffusive permeability rate (Po) (cm/sec) was calculated as previously described (Harhaj et al., 2002).
2.4. Immunofluorescence
BREC monolayers were fixed with 1% paraformaldehyde, permeabilized with 0.2% Triton X-100 and blocked with 10% goat serum, followed by their incubation with primary antibodies: polyclonal rabbit α-Claudin-5 (Invitrogen; 1:100), monoclonal mouse α-Occludin (Invitrogen; 1:100), monoclonal rat α-ZO-1 (Millipore; 1:100), or monoclonal mouse α-VE-Cadherin (F-8) (Santa Cruz Biotechnology; 1:100), for 2 days at 4°C. The primary antibodies were detected using secondary fluorescent antibodies: goat anti-mouse Alexa Fluor 488 (Life Technoligies, 1:400), goat anti-rabbit Alexa Fluor 594 (Life Technologies, 1:400), goat anti-rat Alexa Fluor 647 (Life Technologies, 1:400) and Hoechst (Life Technologies, 1:1000) overnight at 4 °C. Samples were imaged using a confocal microscope (TCS SP5; Leica, Wetzlar, Germany).
2.5. Western blot
Total protein content of confluent BREC monolayers was quantified by Western immunoblotting. BREC were harvested in a detergent-based extraction buffer. After the determination of total protein concentrations using DC protein assay kit (Bio-Rad), fifty micrograms protein was diluted in LDS sample buffer and was loaded on 4%–12% Bis-Tris 1.5-mm, 10-well gels (NuPAGE, Invitrogen). The gels were run according to NuPAGE protocols using MOPS SDS running buffer (Invitrogen), transferred to MSI nitrocellulose (Fisher Scientific, Pittsburgh, PA), blocked in 2% ECL Advance blocking reagent (Invitrogen) in 0.5% TBS-T, and immunoblotted with: polyclonal rabbit α-Claudin-5 (Invitrogen; 1:1000), polyclonal rabbit α-Occludin (Invitrogen; 1:1000), monoclonal rat α-ZO-1 (Millipore; 1:1000), or monoclonal mouse α-β-Actin (Cell Signaling; 1:5000). Primary antibodies were detected with horseradish peroxidase-conjugated α-mouse, α-rabbit or α-rat IgG and chemiluminescence (ECL Plus; GE Healthcare, Buckingham-shire, England). Images were acquired with FluorChem™ E chemiluminescent detector (Protein Simple, San Jose, CA) and the intensity of each band was analyzed using ImageJ 1.46r (National Institutes of Health, USA) software.
2.6. qRT-PCR
RNA from BREC monolayers was extracted and purified from gDNA, with RNeasy Plus mini kit (Qiagen, Gilden, Germany), after 48 hours of stimulation with Shh, cyclopamine, purmorphamine and/or VEGF. 1 μg of RNA per sample was processed with Omniscript Reverse Transcription kit (Qiagen) to obtain cDNA and finally, qPCR was performed with TaqMan® Real-Time PCR master mix (Thermo Fisher Scientific, Waltham, MA) using specific primers to detect Sox18 and Ptch1 gene expression. Results were normalized to β-actin mRNA expression.
2.7. Statistical analysis
Data were analyzed for means and standard errors of the mean (SEM) using GraphPad software (Instat version 6 for Mac OS X, San Diego, California). T-test, one or two way ANOVA tests were performed as indicated in detail at the bottom of each figure legend.
3. Results
3.1. Cyclopamine is additive with VEGF to increase endothelial permeability in vitro
A recent study suggested that the Sonic Hedgehog (Shh) signaling pathway is involved in stabilizing the blood-brain barrier (Alvarez et al., 2011), however its role in maintaining blood-retinal barrier (BRB) is not known and the potential of Shh to reverse vascular endothelial growth factor (VEGF) induced permeability has not been tested. To assess whether Shh signaling affects diffusive permeability of retinal endothelial cells, we used a well-established in vitro model of a primary culture of bovine retinal endothelial cells (BREC). BREC monolayers were stimulated with human recombinant Shh or cyclopamine, a Shh pathway inhibitor through Smoothened (SMO) receptor, separately or in combination with VEGF and diffusive permeability to 70kDa RITC-dextran was assessed. Administration of VEGF induced an increase in permeability of 70 kDa RITC-dextran in comparison with control (*P<0.01), as previously reported (Harhaj et al., 2006), while Shh addition either alone or with VEGF, did not alter the permeability to 70kDa RITC-dextran (Figure1). Interestingly, the addition of VEGF and cyclopamine together induced an additive increase in BREC permeability that was significant in comparison with all conditions (***P<0.0001). This data suggests an endogenous Shh signaling in retinal endothelial cells that contributes to barrier maintenance. Moreover, inhibition of Shh signaling through SMO receptor and VEGF act additively to inhibit BREC monolayer permeability.
Figure 1. VEGF and cyclopamine act additively to increase the permeability of BREC monolayers.

BREC stimulated with vehicle (C), human recombinant sonic hedgehog (Shh; 100 ng/ml), an inhibitor of Shh signaling: cyclopamine (Cyclo; 10 μM), and/or vascular endothelial growth factor (VEGF; 50 ng/ml) in 1% FBS step down medium. After 48 hours, the permeability of a 70kDa RITC-dextran molecule was measured by collecting 50 μl aliquots from basolateral chamber every 30 min for 3.5 hours and diffusive permeability rate was calculated (Po in cm/sec). Basal permeability values were around 5.4×10-7 (100%) and all values were normalized to the control value of each experiment. Data represent the mean ± SEM; P values are shown (* vs C, # vs Shh and *** vs all conditions) as tested by ONE Way ANOVA and multiple comparison, followed by Bonferrioni's post-test; N=7 for each group.
As a complementary assay for BRB integrity, we performed real time measurements of trans-endothelial electrical resistance (TEER) that is sensitive to permeation of ions across the monolayer, using an ECIS Z-theta system. Cells were plated (day 0) and grown on gold-plated electrode arrays and were measured from day 1 with a reading of 200 Ω within 0.8 cm2 growth area and reached a plateau of 2000 Ω 2-days after changing to 1% fetal bovine serum (FBS) media with hydrocortisone (data not shown). To determine if VEGF and Shh act through a common signaling pathway to regulate the barrier properties of BREC, we administered Shh or cyclopamine separately or in combination with VEGF and assayed for changes in TEER. Similar to our diffusive permeability experiments, VEGF reduced TEER to approximately 30% of the control value (blue; Fig. 2A). VEGF and Shh added together yielded results identical to VEGF alone in TEER (red). Administration of Shh (pink) resulted in no change in TEER in comparison with the control monolayer (black). Conversely, cyclopamine significantly decreased TEER of BREC to approximately 50% of control values by 5 h and even faster and to a greater extent than VEGF alone by the first 11 h (dark green). Further, VEGF and cyclopamine demonstrated an additive effect in reducing TEER (light green). These data support a role for cell autonomous Shh signaling in endothelial barrier properties in a signaling pathway separate from VEGF.
Figure 2. VEGF and cyclopamine decrease the TEER of BREC monolayers.

Real time measurements of TEER in BREC treated with vehicle (C), Shh (100 ng/ml), Cyclo (10 μM), and/or VEGF (50 ng/ml) in 1% FBS step down medium. All monolayers showed initial TEER values above 2000 Ω, values from every hour were normalized to the starting point (an hour before treatments). Red dotted line in B, indicates the addition of VEGF after Shh. Data represent the mean + SEM; significant differences are indicated at the top of the graph in colored bars that match with the condition compared vs VEGF, or vs Cyclo in A, and vs VEGF in B; as assessed by TWO Way ANOVA and multiple comparison, followed by Bonferrioni's post-test; N = 4–6 for each group. VEGF-treated monolayers were significant different from control monolayers. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We further tested whether increased time or doses of stimulation with Shh could counter VEGF-induced permeability. The addition of Shh for 4 (light blue) or 24 (orange) hours before addition of VEGF did not prevent VEGF decrease in TEER (Fig. 2B). Likewise, after 30 h incubation with VEGF, BREC monolayer were stimulated with Shh at 10, 30, 100 and 300 ng/ml, resulted in no change in TEER in comparison with VEGF alone (supplemental figure). As a complimentary experiment, we stimulated BREC monolayers with VEGF and 10 or 100 ng/ml of purmorphamine (Purm), a purine-based small molecule agonist of SMO receptor (Sinha and Chen, 2006). However, purmorphamine was not able to restore VEGF decrease in TEER (supplemental figure). This data reveals that recombinant Shh or a chemical agonist of the SMO receptor, are not sufficient to rescue VEGF-mediated BREC permeability.
3.2. Cyclopamine alters tight junction protein localization
Changes in tight junction (TJ) protein expression or organization may contribute to increased permeability. To determine whether cyclopamine altered the expression of TJ proteins, VEGF, recombinant Shh or cyclopamine were added to cultures separately or in combination, and western blot of TJ proteins ZO-1, occludin and claudin-5 were performed. The western blot of claudin-5 and occludin showed no change in total content in the observed time course but ZO-1 decreased after the addition of VEGF and cyclopamine together (Figure 3).
Figure 3. VEGF and cyclopamine additively affect the expression of ZO-1 but not claudin-5 or occludin.

After 48 hours of stimulation, BREC were harvested to be processed for western blot. (A) Representative WB. (B) Quantification of bands intensity normalized to total protein (β-Actin) and to control levels. Data represent the mean ± SEM; P values are indicated as tested by ONE Way ANOVA and multiple comparison, followed by Bonferrioni's post-test; N=4 for each group.
Altered organization and localization of TJ proteins contribute to altered endothelial permeability. To assess whether cyclopamine altered the subcellular localization of ZO-1, occludin and claudin-5, we performed immunofluorescence confocal microscopy of BREC monolayers. Administration of VEGF yielded changes in endothelial cell morphology and modest changes in occludin and ZO-1 localization, while changes in VE-cadherin and claudin-5 were not readily apparent (Figure 4). Addition of Shh did not alter either the basal or VEGF treated samples, since morphology and TJ organization were observed comparable to VEGF treatment alone. Addition of cyclopamine dramatically reduced the occludin and ZO-1 border staining, while the localization of claudin-5 or VE-cadherin was less affected. Concurrent administration of cyclopamine and VEGF showed a further decrease in occludin and ZO-1 staining at the cell border and a dramatic increase in occludin staining as puncta. Furthermore, increased intracellular staining of claudin-5 and VE-cadherin was observed. Together these data suggest that VEGF and cyclopamine decrease BREC monolayer integrity by changing localization of TJ proteins and that VEGF and cyclopamine utilize distinct downstream pathways in affecting BREC monolayer barrier properties.
Figure 4. Cyclopamine decreases occludin and ZO-1 localization at the cell borders and promotes claudin-5 and VE-Cadherin intracellular staining in BREC.

BREC treated with vehicle (Control), Shh (100 ng/ml), Cyclo (10 μM), and/or VEGF (50 ng/ml) in 1% FBS step down medium for 48 hours, were analyzed for immunoreactivity of claudin-5, occludin and ZO-1 (A) or VE-cadherin and ZO-1 (B). In each condition, images of four random fields from four independent experiments were taken and the representative images are shown. Scale bar, 25 μm.
3.3. Cell Autonomous Shh signaling in BREC
Since cyclopamine reduced endothelial barrier properties but addition of Shh had no effect, we hypothesized that cells may activate Shh signaling in a cell autonomous fashion. To address this possibility, BREC monolayers were stimulated with Shh, cyclopamine, purmorphamine and/or VEGF. After 48 h, the RNA from monolayers was extracted to measure the expression of genes activated downstream of the SMO/Gli pathway, such as Sox18 and Ptch1. As shown in Fig. 5, Sox18 and Ptch1 demonstrated basal expression that was not increased by the addition of and Shh or purmorphamine, but cyclopamine significantly decreased expression of these genes in a manner independent of Shh or purmorphamine addition, suggesting that Shh pathway is already activated in BREC monolayers. VEGF only decreased Ptch1 gene expression and the addition of Shh was not sufficient to restore Ptch1 expression in monolayers stimulated with VEGF. Interestingly, the addition of Shh, cyclopamine or purmorphamine with VEGF had a slight but significant effect in Sox18 and Ptch1 gene expression beyond that of VEGF.
Figure 5. VEGF and cyclopamine decrease the expression of Gli targets.
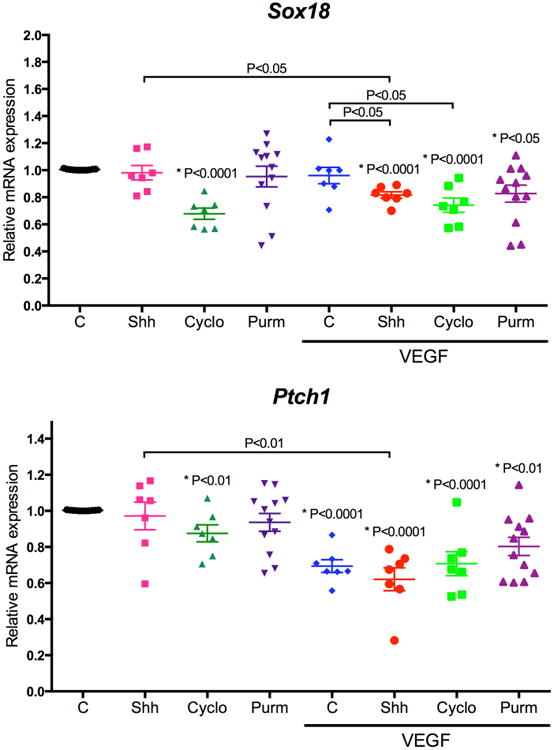
After 48 h of stimulation, RNA from BREC monolayers was extracted for RT-qPCR of specific genes. Quantification of gene expression normalized to the non-stimulated control and to β-Actin mRNA. Data represent the mean ± SEM; P values are indicated as tested by Student t-test compared to untreated control (*) or the condition indicated; N = 7–12 for each group.
In order to determine whether cyclopamine's effects on BREC monolayer integrity is through Shh signaling versus non-specific or off target effects, we also tested another Shh pathway antagonist, Vismodegib (Vismo), and its effects on endothelial permeability (Taipale et al., 2000). Vismodegib, or Erivedge (also known as GDC-0449) is an FDA approved drug for the treatment of advanced basal cell carcinomas (BCC) and specifically binds and inhibits SMO receptor (Wahid et al., 2016). Continuous measurements of TEER on BREC monolayers stimulated with Vismodegib, VEGF or both, showed that Vismodegib significantly decreased TEER control values by 33 h (pink) and that this effect was additive to VEGF stimulation by 27 h (light blue; Fig. 6); however, the effect size was significantly less than with cyclopamine. These results support the hypothesis that Shh/SMO signaling pathway regulates BREC permeability in a cell autonomous manner and independent of VEGF signaling.
Figure 6. Vismodegib decrease the TEER of BREC monolayers.

Real time measurements of TEER in BREC stimulated simultaneously with vehicle (C), an antagonist of Shh signaling: vismodegib (Vismo, 200 mg/ml) and/or VEGF (50ng/ml) in 1% FBS. Data represent the mean + SEM; significant differences are indicated at the top of the graph in colored bars that match with the condition compared vs Control, or vs VEGF; as assessed by TWO Way ANOVA and multiple comparison, followed by Bonferrioni's post-test; N=6 for each group. VEGF-treated monolayers were significant different from control monolayers.
4. Discussion
In this study, we were able to demonstrate a novel cell autonomous role for the Shh pathway in regulating endothelial permeability in an in vitro model of retinal vascular endothelial cells that appears to act in parallel to VEGF-signaling pathways. Our results showed that VEGF and SMO inhibitors had profound effects on tight junctions structure that involved redistribution of key proteins. It should be noted that inhibition with cyclopamine was much more pronounced than with Vismodegib, suggesting that cyclopamine may have additional off target effects. However, the regulation of known Gli targets and the observed altered barrier properties with both cyclopamine and Vismodegib provide evidence for the role of SMO signaling in primary endothelial cell culture.
This research identified a role for SMO signaling in regulating retinal vascular permeability consistent with recent studies demonstrating a role for Shh in promoting blood-brain barrier integrity (Alvarez et al., 2011) and add to these data by demonstrating SMO inhibition directly inhibits barrier properties, even in the absence of pericytes or glial cells. Here we demonstrate that a SMO inhibitor, cyclopamine, decreases BREC monolayer integrity and causes changes of claudin-5, ZO-1, occludin, and VE-cadherin localization. This contrasts with data from human brain endothelial cells treated with cyclopamine and astrocyte conditioned medium (ACM), which showed reduced protein expression levels of claudin-5, occludin, JAM-A, claudin-3, and VE-cadherin by western blot (Alvarez et al., 2011). Interestingly, in an animal model of stroke, recombinant Shh was shown to regulate levels of the TJ protein ZO-1 through regulation of angiopoietin-1 (Xia et al., 2013). In our in vitro model, ZO-1 did not increase with the Shh stimulation, but the addition of VEGF and cyclopamine together decreased total content of this TJ protein.
We did not observe an increase of vascular permeability by administering recombinant Shh or purmorphamine, as we hypothesized. Indeed, the additive nature of cyclopamine or Vismodegib with VEGF on endothelial permeability and TJ organization suggest parallel signaling pathways. Further, in this in vitro system, the BREC exhibit endogenous Shh signaling, such that cyclopamine reduces SMO targeted gene expression but recombinant Shh or purmorphamine did not increase the SMO signaling pathway. These data are consistent with a recent report that shows that BREC monolayers express hedgehog proteins (Walshe et al., 2011).
A previous report suggested that cyclopamine could inhibit neovascularization in both an oxygen-induced retinopathy model as well as a laser-induced choroid neovascularization model, suggesting that the Shh pathway normally promotes neovascularization (Surace et al., 2006). As neovascular vessels are often associated with breakdown of the BRB, this finding suggests a complex role of Shh signaling in vessel growth and barrier formation potentially pointing to separate roles in angiogenesis and barrier formation.
5. Conclusions
This work has identified a novel cell autonomous role of Shh/SMO signaling in maintenance of BRB integrity in primary retinal endothelial cells distinct from VEGF signaling. Future work will help to elucidate the downstream factors that mediate the SMO pathway regulation of barrier properties, and how these signaling pathways interact in the presence of contributions from pericytes and glial cells to induce and maintain the BRB in Vivo.
Supplementary Material
Real time measurements of TEER in BREC stimulated with vehicle (C), Shh signaling agonists: Shh (10, 30, 100 and 300 ng/ml) or purmorphamine (Purm; 10 and 100 ng/ml) and/or VEGF in 1% FBS. Red dotted line indicates the addition of Shh or Purm after VEGF. Data represent the mean + SEM.
Highlights.
Cell autonomous basal activity of hedgehog signaling in retinal endothelial cells.
Endogenous hedgehog signaling is required for endothelial barrier maintenance.
Inhibition of hedgehog signaling with cyclopamine leads to loss of barrier properties.
Cyclopamine effect is additive with VEGF to increase endothelial permeability.
Acknowledgments
This work was supported by the National Eye Institute P30 EY014801 (University of Miami), an ASCRS Foundation Research Grant (DLC), NIH EY012021 (DAA), and Kellogg Eye Center Core Center for Vision Research, NIH P30EY007003 (Bret Hughes, Ph.D., PI), and unrestricted grants from Research to Prevent Blindness, Inc (Universities of Miami, Michigan).
Footnotes
Disclosures: MDC: None
DLC: None
ES: None
JLG: None
DAA: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonnière L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–1731. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, VG HD, Lin CM. Vascular Permeability in Diabetic Retinopathy. In: Duh EJ, editor. Diabetic Retinopathy. Humana Press; Totowa, New Jersey: 2008. [Google Scholar]
- Antonetti DA, Wolpert EB. Isolation and characterization of retinal endothelial cells. Methods Mol Med. 2003;89:365–374. doi: 10.1385/1-59259-419-0:365. [DOI] [PubMed] [Google Scholar]
- Aveleira CA, Lin CM, Abcouwer SF, Ambrosio AF, Antonetti DA. TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes. 2010;59:2872–2882. doi: 10.2337/db09-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- Frey T, Antonetti DA. Alterations to the blood-retinal barrier in diabetes: cytokines and reactive oxygen species. Antioxid Redox Signal. 2011;15:1271–1284. doi: 10.1089/ars.2011.3906. [DOI] [PubMed] [Google Scholar]
- Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:772–783. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- Günzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013;93:525–569. doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harhaj NS, Barber AJ, Antonetti DA. Platelet-derived growth factor mediates tight junction redistribution and increases permeability in MDCK cells. J Cell Physiol. 2002;193:349–364. doi: 10.1002/jcp.10183. [DOI] [PubMed] [Google Scholar]
- Harhaj NS, Felinski EA, Wolpert EB, Sundstrom JM, Gardner TW, Antonetti DA. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest Ophthalmol Vis Sci. 2006;47:5106–5115. doi: 10.1167/iovs.06-0322. [DOI] [PubMed] [Google Scholar]
- Jenkins D. Hedgehog signalling: emerging evidence for non-canonical pathways. Cell Signal. 2009;21:1023–1034. doi: 10.1016/j.cellsig.2009.01.033. [DOI] [PubMed] [Google Scholar]
- Krug SM, Amasheh S, Richter JF, Milatz S, Günzel D, Westphal JK, Huber O, Schulzke JD, Fromm M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell. 2009;20:3713–3724. doi: 10.1091/mbc.E09-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase T, Nagase M, Machida M, Fujita T. Hedgehog signalling in vascular development. Angiogenesis. 2008;11:71–77. doi: 10.1007/s10456-008-9105-5. [DOI] [PubMed] [Google Scholar]
- Runkle EA, Antonetti DA. The blood-retinal barrier: structure and functional significance. Methods Mol Biol. 2011;686:133–148. doi: 10.1007/978-1-60761-938-3_5. [DOI] [PubMed] [Google Scholar]
- Shen W, Fruttiger M, Zhu L, Chung SH, Barnett NL, Kirk JK, Lee S, Coorey NJ, Killingsworth M, Sherman LS, Gillies MC. Conditional Müllercell ablation causes independent neuronal and vascular pathologies in a novel transgenic model. J Neurosci. 2012;32:15715–15727. doi: 10.1523/JNEUROSCI.2841-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Chen JK. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat Chem Biol. 2006;2:29–30. doi: 10.1038/nchembio753. [DOI] [PubMed] [Google Scholar]
- Surace EM, Balaggan KS, Tessitore A, Mussolino C, Cotugno G, Bonetti C, Vitale A, Ali RR, Auricchio A. Inhibition of ocular neovascularization by hedgehog blockade. Mol Ther. 2006;13:573–579. doi: 10.1016/j.ymthe.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Malik AB, Del Vecchio PJ, Keese CR, Giaever I. Electrical method for detection of endothelial cell shape change in real time: assessment of endothelial barrier function. Proc Natl Acad Sci U S A. 1992;89:7919–7923. doi: 10.1073/pnas.89.17.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traiffort E, Angot E, Ruat M. Sonic Hedgehog signaling in the mammalian brain. J Neurochem. 2010;113:576–590. doi: 10.1111/j.1471-4159.2010.06642.x. [DOI] [PubMed] [Google Scholar]
- Wahid M, Jawed A, Mandal RK, Dar SA, Khan S, Akhter N, Haque S. Vismodegib, itraconazole and sonidegib as hedgehog pathway inhibitors and their relative competencies in the treatment of basal cell carcinomas. Crit Rev Oncol Hematol. 2016;98:235–241. doi: 10.1016/j.critrevonc.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Walshe TE, Connell P, Cryan L, Ferguson G, Gardiner T, Morrow D, Redmond EM, O'Brien C, Cahill PA. Microvascular retinal endothelial and pericyte cell apoptosis in vitro: role of hedgehog and Notch signaling. Invest Ophthalmol Vis Sci. 2011;52:4472–4483. doi: 10.1167/iovs.10-7061. [DOI] [PubMed] [Google Scholar]
- Xia YP, He QW, Li YN, Chen SC, Huang M, Wang Y, Gao Y, Huang Y, Wang MD, Mao L, Hu B. Recombinant human sonic hedgehog protein regulates the expression of ZO-1 and occludin by activating angiopoietin-1 in stroke damage. PLoS One. 2013;8:e68891. doi: 10.1371/journal.pone.0068891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Real time measurements of TEER in BREC stimulated with vehicle (C), Shh signaling agonists: Shh (10, 30, 100 and 300 ng/ml) or purmorphamine (Purm; 10 and 100 ng/ml) and/or VEGF in 1% FBS. Red dotted line indicates the addition of Shh or Purm after VEGF. Data represent the mean + SEM.


