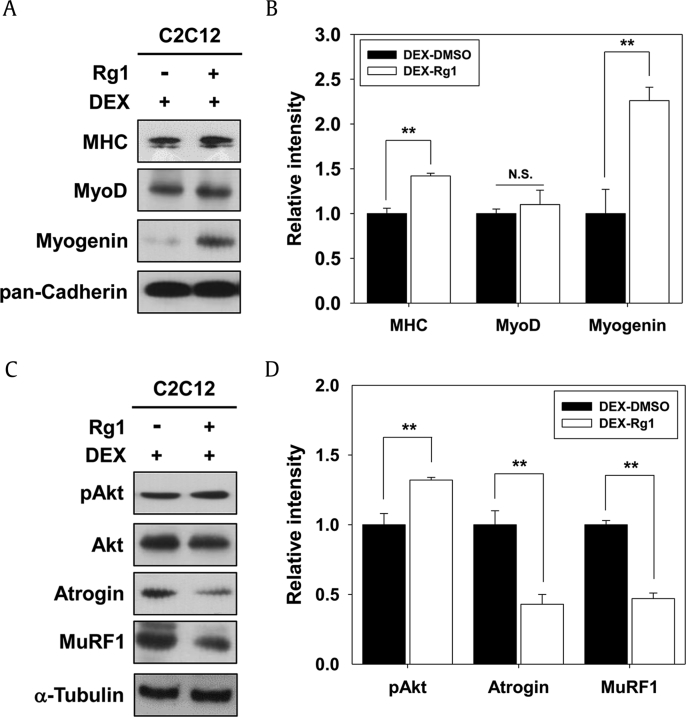
Fig. 4.
Rg1 prevented DEX-induced myotube atrophy through activation of Akt signaling. (A) C2C12 cells were differentiated in DM for 1 d and then treated with 25μM DEX along with vehicle or 10nM Rg1 in fresh DM for additional 2 d. The cell lysates were followed by immunoblotting against antibodies of muscle-specific proteins. Pan-Cadherin was used as the loading control. (B) The signal intensity of indicated muscle-specific proteins was quantified in three independent experiments and normalized to the loading control pan-Cadherin. The values from DMSO-treated DEX-induced cells were set to 1.0. Values are presented as means ± SD. ** p < 0.01 compared to control. (C) Cell lysates were subjected to immunoblotting against anti-pAkt, anti-Akt, anti-Atrogin, and anti-MuRF1. α-Tubulin was used as the loading control. (D) Quantification of three experiments was performed as shown in panel C. The signal intensity of phosphorylated Akt was calculated and normalized to the loading control of total Akt. The signaling intensities of Atrogin and MuRF1 proteins were calculated and normalized to the loading control α-Tubulin. The values from DMSO-treated DEX-induced cells were set to 1.0. Values are presented as means ± SD. ** p < 0.01 compared to control group. DEX, dexamethasone; DM, differentiation medium; DMSO, dimethyl sulfoxide; MHC, myosin heavy chain; NS, not significant; SD, standard deviation.