Abstract
Operculina turpethum (Linn.) (OT) Silva Manso belongs to the family Convolvulaceae. This review incorporates literature for the phytochemical and pharmacological profile of OT herb. Exhaustive literature survey was done using all the details on phytochemistry and pharmacology of OT available. This herb was found to be a potent source of bioactive compounds such as α- and β-turpethein, turpethinic acids (A, B, C, D, and E), coumarins, cycloartenol, lanosta-5-ene, 24-methylene-δ-5-lanosterol, α- and β-rhamnose, β-sitosterol, lupeol, scopoletin, betulin, acrylamide, stigma-5,22dien-3-O-β-D-glucopyranoside, β-sitosterol-β-D-glucoside (H-1), 22,23-dihydro-α-spinosterol-β-D-glucoside (H-2), and salicylic acid (CH-2), which are useful in fevers, edema, ascites, anorexia, constipation, hepatosplenomegaly, hemorrhoids, cervical lymphadenitis, fistulas, constipation, chronic gout, fever, bronchitis, ulcers, hemorrhoids, tumors, obesity, jaundice, herpes, induce lacrimation, and other skin disorders. From the aerial parts of OT, four new dammarane-type saponins that are operculinosides A–D (1–4) were isolated that showed particular hepatoprotective activities. All the compounds are reported to possess pharmacological properties such as antibacterial, anti-inflammatory, analgesic, hepatoprotective, anti-arthritic, ulcer protective, antidiarrheal, antidiabetic, and cytotoxic properties.
Keywords: Convolvulaceae, Operculina turpethum, operculinosides, turpethinic acids
INTRODUCTION
Operculina turpethum (Linn.) (OT) Silva Manso also called as Tihudi/Trivrit because of the triangular shape stem is an Indian Ayurvedic herb, belonging to the family Convolvulaceae. It is known by other names Indian Jalap/Turpeth root in English, Nisoth/Turpeth/Panila/Pithori in Hindi, Trivrit in Sanskrit, Tegada in Telugu, Trikolpakkonna/Triputa/Sivata in Malayalam, Sigade in Kannada, and Kumbham/Sivatai in Tamil. It is usually found on the roadsides across India, up to 1000 square feet and sometimes cultivated in gardens as an ornament. The distribution of this plant is randomly found in tropical regions of India, America, Pakistan, Sri Lanka, China, Philippines, Bangladesh, Madagascar, Mauritania, and Africa.[1]
The root bark of the plant contains a glycosidic resin, which has the insoluble glycoside turpethein. It also contains a large number of secondary metabolites including saponins, flavonoids, glycosides, and phenolics as well as some amount of essential oil, glucose and fructose.[2] Chemical constituents of OT are resins, a mixture of α- and β-turpethein, glycosides, coumarins, scopoletin, saponins flavonoids, steroids, and carbohydrates.[1,3,4,5] It comprises a wide variety of phytoconstituents including glycosidic resin, coumarin, beta-sitosterol, reducing sugars, and essential oils, which are helpful for the treatment of various ailments/diseases.[5,6,7,8]
Since it implies as a potent medicinal plant, it is also employed for several medicinal purposes. Often, the root bark and seed of this herb are used in the Ayurvedic system of medicine for the treatment of skin disorders such as vitiligo and several diseases such as cervical lymphadenitis, fistulas, constipation, chronic gout, fever, bronchitis, ulcers, hemorrhoids, tumors, obesity, jaundice, herpes, and induced lacrimation. The use of root powder for the treatment of rheumatism, flatulence, paralysis, scorpion sting, and snake bite was also proven.[2,9,10,11] The root powder was also found to be useful for the treatment of hematemesis, herpes, and tuberculosis, and for the treatment of corneal opacity and conjunctivitis, fresh juice of leaves is employed.[12] A significant Ayurvedic formulation of Trivrit (OT) is Avipattikar Churna which is used predominantly in disorders pertaining of stomach and intestines.
In Ayurvedic system, OT has been incorporated as in the group of “ten purgative herbs,” supportive for a therapeutic enema,[13] ten antidote herbs in the group of herbs eliminating the toxins, and in the group of colon cleanser, anticancer, and antidote herbs.[14] Young leaves and stem of the plant are employed as a vegetable.[15] The antimicrobial activity of the stem was also studied.[16]
TAXONOMICAL CLASSIFICATION
PARTS USED
Apart from the whole plant, seeds, root bark, root, stem, and leaves are also used.
SYNONYMS
Convolvulus turpethum (L.) (Basionym); Ipomoea turpethum (L.), Merremia turpethum (L.), Spiranthera turpethum (L.) (Homotypic); Convolvulus triqueter (L.), Convolvulus anceps (L.), Ipomoea anceps (L.), Ipomoea diplocalyx (L.), I. turpethum var. anceps (L.), Ipomoea silvana (L.), OT var. heterophylla Hallier F (L.), Operculina triquetra (L.), OT var. ventricosa, Argyreia alulata (L.) (Heterotypic).[17,19]
AYURVEDIC DESCRIPTION
Sanskrit name: Shweta (white); Tribhandi, Trivrit, Triputa (stem is triangular in shape); Sarala, Suvaha, Rechani (causes purgation); Sarvanubhuti, Nishotra, Kalaparni, Nandi, Kalameshi, Kutarana, Bhandi, Palindi, Ardhachandra, Sushenika, Masurvidala, Kaulkaushiki, Kalameshika, Shyama[12]
Properties: Rasa (taste) – Madhura (sweet), Titka (bitter), Katu (pungent), Kashaya (astringent); Guna (qualities) – Laghu (light), Rooksha (dry), Teekshna (strong); Vipaka (effect) – Katu; Veerya (potency) – Ushna (hot)[20]
Actions: Sukhavirechan, bhedaniya (purgation action), balances Pitta and Kapha, increases Vata dosha
Therapeutic uses: Virechana/Panchakarma, Jvaraghna (fever), Krumihara (useful in worm infestation, infected wounds), Shleshmodara (useful in ascites), Shophahara (useful in inflammation), Panduhara (useful in anemia, liver disorders), Pleeha (splenomegaly), Hrudroga (useful in heart diseases), Vatasruk (useful in gout), Udavartahara (relieves bloating, gas distension in abdomen).[21]
GROWTH AND DISTRIBUTION
There are two kinds of Trivrit, named as Shweta or white Turpeth (the botanical name is OT Silva Manso, Synonym. I. turpethum) and Krishna or black Turpeth (the botanical name is Ipomoea petaloidea Chois).[22] OT grows throughout India at an altitude of 900 m. It is a native herb and scattered in Australia (Northern Territory, Queensland); Africa (Kenya, Tanzania, Mozambique, Zimbabwe; Western Indian Ocean: Madagascar, Mauritius, Reunion); Pacific (Northwestern Pacific: Micronesia); Asia-Tropical (India, Nepal, Pakistan, Sri Lanka, Indochina, Myanmar, Thailand, Indonesia, Malaysia, Papua New Guinea, Philippines); and Asia-Temperate (China: China-Guangdong; Eastern Asia: Taiwan); on the other hand, it is naturalized in Southern America (West Indies).[17,18,23,24,25,26,27]
PHYTOCHEMICAL PROFILE
Mhaskar et al.[28] and Rastogi et al.[29] reported that OT (Trivrit) contains turpethinic acids (A, B, C, D, and E) in a large quantity,[23] which were isolated from the resin of the plant and also contain different types of bioactive compounds such as albumin, lignin salts, volatile oil, starch, ferric oxide, lupeol, α- and β-turpethein, α- and β-rhamnose, fructose, β-sitosterol [Figure 1], scopoletin [Figure 2], betulin [Figure 3], and some ether-soluble resin.[9,30,31]
Figure 1.

β-sitosterol
Figure 2.

Scopoletin
Figure 3.
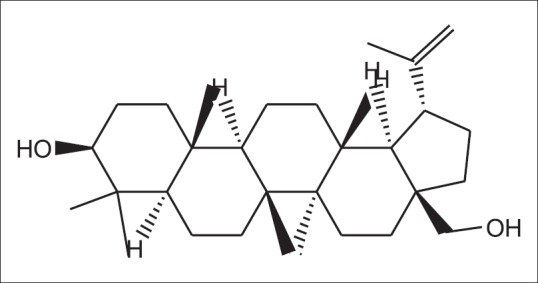
Betulin
Some triterpenoids such as cycloartenol [Figure 4], lanosta-5-ene [Figure 5], and 24-methylene-δ-5-lanosterol and turpethosides were also isolated from the roots of OT.[32,33,34] Therapeutically, terpenoids are reported to exhibit antiviral, antibacterial, antitumor, antiseptic, diuretic, and analgesic activities.[35]
Figure 4.
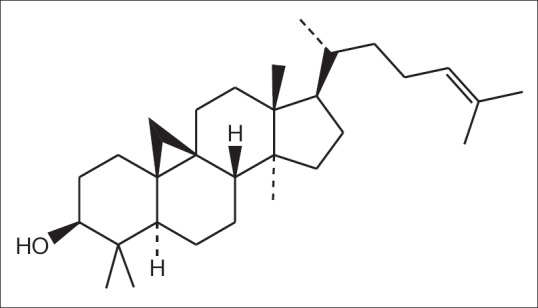
Cycloartenol
Figure 5.
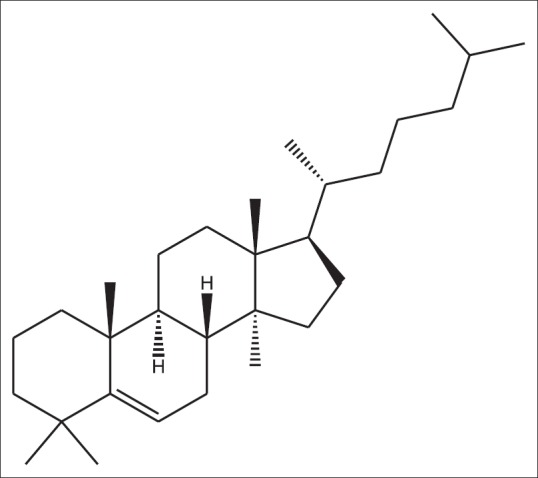
Lanosta-5-ene
The root bark of OT also contains almost 10% “turpethein” (Turpeth resin) of the total phytoconstituents, which is a glycoside analog of Jalapine and Convolvulin. The laxative effect of Trivrit is mainly due to the presence of turpethein,[2,9,28] and it is insoluble in ether, benzene, essential oils, and carbon sulfide but soluble in alcohol. On treating with hydrochloric acid, it is transformed into turpetholic acid, glucose, and fructose, and on treating with alkaline bases, turpethein is changed into turpethic acid. The leaf of the plant is rich in secondary metabolites such as flavonoids, cardiac glycosides, and terpenoids. The roots have various characteristics such as bitter, acrid, sweet, thermogenic, analgesic, purgative, carminative, anthelmintic, expectorant, antipyretic, hepatic, stimulant, and hydragogue.[36]
Veena and Manu in 2013a[37] reported that several phenolic compounds such as tannins present in the cells of plants are potent inhibitors of many hydrolytic enzymes such as proteolytic macerating enzymes employed by plant pathogens. Several plants contain nontoxic glycosides that can get converted into phenolics by hydrolysis which is toxic to microbial pathogens.[38]
Jalaj and Madhavan[39] in 2016 carried out an experiment to identify and quantify phenolic compounds present in methanolic leaf extract of OT by high-performance liquid chromatography (HPLC), and thus, total phenol (1.89 mg/mL) and flavonoid contents (1.0 mg/mL) were estimated from the methanolic leaf and stem extract according to the standard procedures.[40,41] Huang et al. in 2010 reported that the natural phenolic compounds possess antioxidant, anticarcinogenic, and anti-inflammatory effects.[42]
Attele et al. in 1999[43] and Veena and Manu in 2013a[37] reported that the plant contains saponins, a particular class of glycosides, and is responsible for a wide variety of pharmacological effects such as anti-allergic, analgesic, anti-inflammatory, antidiabetic, bactericidal, antifungal, antiviral, and cytotoxic.[44,45] Gopish and Kannabiran in 2008 reported the presence of antimicrobial action of saponins of OT against many microorganisms.[46]
The qualitative phytochemical screening was done by Arif et al. in 2013[47] for the presence of various phytochemicals in the different extracts of OT. They reported that carbohydrates, steroids, gums, and saponins are present in the ethanolic root extracts of OT; carbohydrates, steroids, gums, flavonoids and saponins are found in the ether and chloroform root extracts of OT; and carbohydrates, steroids, gums, flavonoids, tannins, alkaloids, reducing sugars and saponins are present in the ethanolic, ether, and chloroform leaf extracts of OT.
Phytochemical constituents such as flavonoids, alkaloids, tannins, and various other aromatic compounds of this plant act as defense mechanisms against many bacterial and fungal pathogens. Doss et al. in 2009,[48] Doss et al. in 2011a,[49] and Doss et al. in 2011b[50] studied and reported that the antibacterial activity of flavonoids is due to their capability to complex with cell walls of bacteria and to complex with extracellular and soluble proteins. Flavonoids are also known to inhibit production of heat shock proteins in various malignant cell lines including leukemia and cancer of breast and colon.[51]
Veena and Manu in 2013a[37] also carried out an experiment in which aqueous extract of OT displayed that flavonoids are present in this herb and could be used for anti-inflammatory and analgesic effects. The presence of alkaloid in the plant denotes that the plant extracts could be employed for antifungal and antiparasitic properties.[52] Verpoorte has studied about 300 alkaloids displaying such properties in 1998.[53] Slobodníková et al. in 2004,[54] Duraiswamy et al. in 2006,[55] and Li et al. in 2007[56] studied and found the same results on the antibacterial effect of related species of the genus Mahonia.
The crude ethanol extract of OT black variety was tested for the presence of several phytochemical classes of compounds such as anthraquinones, terpenoids, tannins, saponins, flavonoids, alkaloids, ketones, sugars, glycosides, steroids, and amino acids.[57]
Basak and Mohapatra in 2015[58] carried out a study and determined coumarin content from wild root barks of OT in the range of 0.212%–0.271% dry weight, and from uncultivated seeds, coumarin content ranged from 0.061% to 0.21% dry weight.
The chemical constituents of OT constitute an extensive array of biochemical and pharmacological activities including anticancer, antioxidant,[59] anti-inflammation, anti-HIV, antibacterial, anticoagulant,[60] antitumor, analgesic,[61,62] and immunomodulation activities.[63,64] The antioxidant, hematopoietic, hepatoprotective, antiulcer, antimicrobial, and antidiabetic activities of OT have been reported.[10,65,66,67,68,69]
Coumarins [Figure 6], commonly known as benzopyrones (2H-1-benzopyran-2-one) present in OT, are composed of fused benzene and α-pyrone rings.[70] Plants produce them as defense substances when invaded or affected by other organisms, so these are designated as phytoalexins.[63] Coumarins serve as antimicrobial, central nervous system activators, antitumor agents, anti-HIV agents, and enzyme inhibitors.[71] The biological activities of coumarins are depicted in Table 1.
Figure 6.

Coumarin [2H-1-benzopyran-2-one]
Table 1.
Biological/medicinal importance of coumarin showed in literature

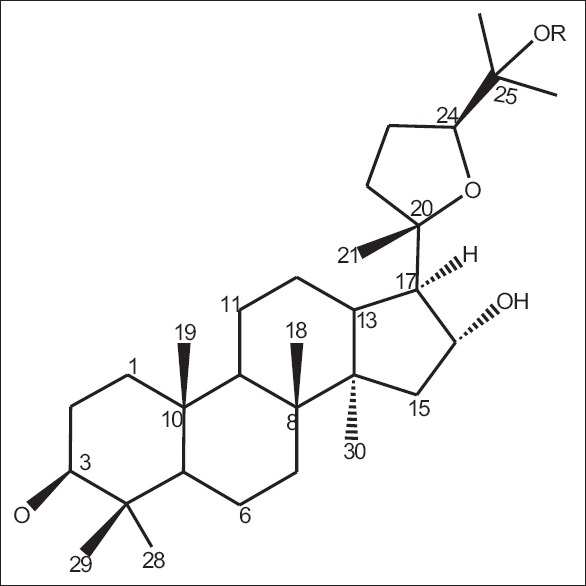
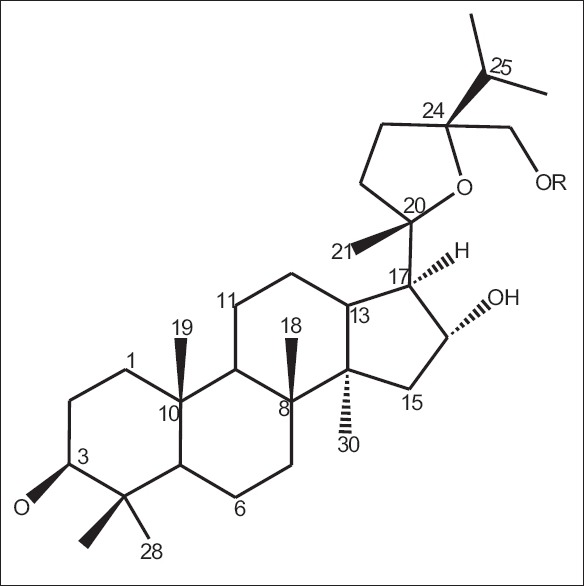
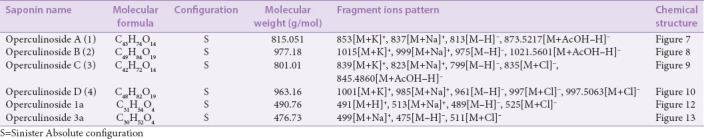
On literature survey, it was found that Xiaoyi et al. in 2011 investigated that from the aerial parts of OT, four new dammarane-type saponins that are operculinosides A–D (1–4) [Figures 7-10] were isolated. Compounds A (1) [Figure 7] and B (2) [Figure 8] are the first two dammarane-type triterpenoids containing an oxymethyl group at C-24, while compounds C (3) [Figure 9] and D (4) [Figure 10] include a β-glucopyranosyl group at C-25. Compounds A (1) and C (3) showed specific hepatoprotective activities against D-galactosamine-induced hepatopathy (liver toxicity) in L-02 human hepatic cells. Using spectroscopic analysis and acid hydrolysis method, the structures of these were determined. The absolute configuration of operculinoside A (1) was confirmed by X-ray crystallographic technique [Figure 11].[33] The chemical parameters of four new dammarane-type saponins, operculinosides A–D (1–4) are presented in Table 2.
Figure 7.
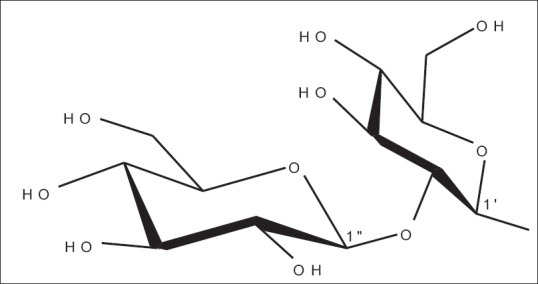
Operculinoside A (1) R = H
Figure 10.
Operculinoside C (4) R=β-Glc
Figure 8.
Operculinoside A (2) R=β-glucopyranose
Figure 9.
Operculinoside C (3) R = H
Figure 11.

X-ray crystallographic structure of operculinoside A (1) according to Xiaoyi et al., 2011
Table 2.
The chemical parameters of four new dammarane-type saponins, operculinosides A–D (1-4) (Xiaoyi et al., 2011)
In an experimental study, the presence of an acrylamide, i.e. 3-(4-hydroxy-phenyl)-N-[2-(4-hydroxy phenyl)-ethyl]-acrylamide [Figure 14] was described by Rashid et al. in 2002, and from the ethyl acetate portion of the stem extracts of I. turpethum (Synonym - OT), it was isolated. They explained its chemistry with spectral analysis.[16]
Figure 12.

Operculinoside 1a R1 = CH2OH, R2 = H
Figure 13.

Operculinoside 3a R1 = H, R2 = OH
Figure 14.
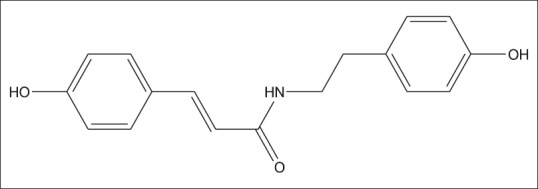
3-(4-hydroxy-phenyl)-N-[2-(4-hydroxy-phenyl)-ethyl]-acrylamide
In another experimental study, Sharma and Singh in 2013c isolated a steroidal glycoside, i.e., stigma-5,22dien-3-O-β-D-glucopyranoside [Figure 15] from the ethanolic extracts of root bark of OT using thin layer chromatography, column chromatography, and HPLC, and the structure of the isolated compound was confirmed by spectroscopic analysis.[77]
Figure 15.
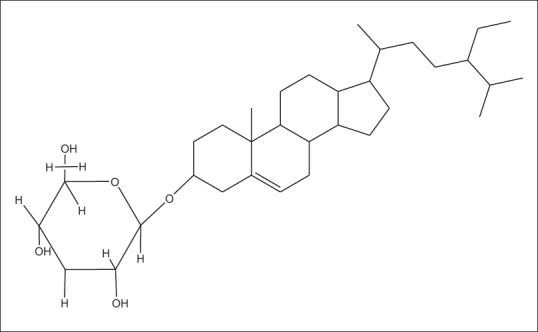
Stigma-5,22dien-3-O-β-D-glucopyranoside
PHARMACOLOGICAL PROFILE
Antimicrobial activity (antibacterial activity)
The antimicrobial agents derived from plant extracts cause leakage from the interior of cells by tampering with the function of the membranes or hindering with intermediary metabolisms or DNA/RNA synthesis/function and thus prevent the bacterial cell wall or protein synthesis.
Alam et al. in 2010 determined the antimicrobial efficacy of the petroleum ether and ethanolic extracts of leaves of OT for their antimicrobial activity. The antimicrobial activity was evaluated by standard disc diffusion method against Gram-positive bacteria such as Streptococcus haemolytica and Bacillus subtilis and Gram-negative bacteria such as Pseudomonas aeruginosa, Shigella sonnei, and Shigella dysenteriae. The ethanolic extract revealed a significant zone of inhibition in various human pathogenic organisms, and thus, the minimum inhibitory concentration (MIC) was found which ranged from 0.13–0.75 mg/mL while petroleum ether extract did not reveal any significant zone of inhibition.[10]
In another study, Srivastava in 1984 and Bauer et al. in 1951 observed the antibacterial potential in I. turpethum using the standard disc diffusion method. The crude extracts of this plant prepared in petroleum ether, chloroform, and ethyl acetate, and three compounds H-1 [Figure 16], H-2 [Figure 17], and CH-2 [Figure 18], viz., β-sitosterol-β-D-glucoside; 22,23-dihydro-α-spinosterol-β-D-glucoside, and salicylic acid (2-hydroxy benzoic acid), respectively, isolated from chloroform stem extract of this plant were employed against 13 human pathogenic bacteria, out of which six bacteria were Gram negative and seven Gram positive. These were assembled from the Institute of Nutrition and Food, University of Dhaka, and International Centre for Diarrhoeal Disease Research, Bangladesh. All extracts and isolated compounds were solubilized in methanol at a concentration of 200 and 100 μg/10 μL, respectively. Nutrient broth and nutrient agar were employed as bacteriological media. Kanamycin was used as standard drug. The bacteriotoxic action of these samples was examined against all the pathogenic bacteria, and by comparing with the approved kanamycin disc (K-30 μg/disc), it was recommended that the bioactive compounds of this plant could be employed as antibacterial agents.[78]
Figure 16.
H-1 (β-sitosterol-β-D-glucoside)
Figure 17.
H-2 (22,23-dihydro-α-spinosterol-β-D-glucoside)
Figure 18.
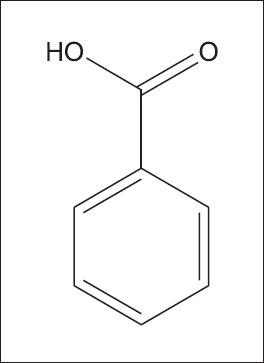
CH-2 (salicylic acid or 2-hydroxy benzoic acid)
Reiner in 1982 measured the MIC of pure compound CH-2 (salicylic acid or 2-hydroxy benzoic acid) [Figure 18] against two Gram-positive and two Gram-negative bacteria, viz., Bacillus subtilis and Sarcinalutea and Escherichia coli and Shigella dysenteriae, respectively, by serial dilution method (107 cells/mL).[79]
In another experimental study, Rashid et al. in 2002 investigated that the crude extracts and isolated compound CH-2 (salicylic acid or 2-hydroxy benzoic acid) [Figure 18] of OT exhibited a significant bacteriotoxic potential with less potent than that of kanamycin while compounds H-1 [Figure 16] and H-2 [Figure 17] presented very fewer effects for microbial infection.[16]
Antihepatotoxic activity
Most of the hepatotoxic chemicals damage liver cells mainly by inducing lipid peroxidation and other oxidative damages in the liver or by forming the reactive free oxygen radicals which directly causes hepatotoxicity or increasing the apoptosis or reducing glutathione stores an oxidant of the human body.
Kumar et al. in 2006 estimated the hepatoprotective activity of OT in paracetamol-induced hepatopathy (liver toxicity) in rats that causing acute centrilobular necrosis. The ethanolic extract of OT administered intraperitoneally at the dose of 100–2000 mg/kg body weight which revealed significant hepatoprotective activity in a dose-dependent manner. Here, silymarin was employed as a standard drug and thus showed a significant increment in hepatoprotective efficacy.[66]
Vaidya et al. in 2010 carried out three experimental analysis and assessed the hepatoprotective action of oral administration of the herbomineral formulation of OT root powder in carbon tetrachloride-induced hepatotoxicity in rats.[80]
Antinephrotoxic activity
Sharma and Singh in 2012a assessed the therapeutic antinephrotoxic potential of a steroidal glycoside, stigma-5,22dien-3-O-β-D-glucopyranoside [Figure 15] in N-nitrosodimethylamine-induced renal carcinogenesis in male mice and hepatotoxicity in the liver of mice. The steroidal glycoside was isolated from the ethanolic fraction of root bark extracts of OT. When the ethanolic extract of the roots and also the isolated compound was administered to mice at 400 and 50 mg/kg doses, respectively, it revealed a significant reduction of nephrotoxicity and hepatopathy.[2]
Antiulcer activity
In an experimental study, the antiulcer activity of methanolic extracts of the stem of OT (MEOTS) and hydroalcoholic extracts of the stem of OT was assessed in aspirin and pyloric ligation-induced ulcer in male albino rats. It was studied by Ignatius et al. The MEOTS and hydroalcoholic stem extracts of OT administered at a dose of 100 mg/kg of body weight which showed a significant antiulcer activity. Ranitidine was used as a standard drug. Finally, it was observed that the hydroalcoholic stem extracts revealed better results than the methanolic extracts.[81]
Rajashekar et al. in 2006 evaluated the ulcer protective effects of OT and its polyherbal formulation through an experiment in rats model found that reduce hyperacidity, gastric ulcer, and gastrointestinal tract-related problems.[82]
Antidiarrheal activity
An experimental study was carried out by Shareef et al. to investigate the antidiarrheal potency of ethanolic root extract of OT through castor oil-induced diarrhea model in mice. Loperamide was used as a standard dose of 10 mg/kg. The root extract was administered orally in three different doses form, and it was observed that the ethanolic root extracts showed antidiarrheal effects in a dose-dependent manner.[83]
Antidiabetic activity
Continuous blood glucose monitoring is needed in diabetes patients because it produces the synergistic effect with oral hypoglycemic agents. OT may be altering the pharmacokinetic of glibenclamide. The possible mechanism by which MEOTS and methanolic extracts of OT roots (MEOTR) exert its hypoglycemic effect in diabetic rats may be due to potentiating the insulin release.
A comparative study was done by Pulipaka et al. to assess the antidiabetic effects of MEOTS and MEOTR in streptozotocin-induced diabetes in experimental rat models at a dose of 100 mg/kg of body weight. Glibenclamide was used as a standard drug. The values obtained were compared with the standard. Findings revealed that methanol extracts showed a significant reduction in the fasting glucose level at the end of 21 days.[67]
Cytotoxic activity
Persoone in 1980, Meyer et al. in 1982, and Mclaughlin and Anderson in 1988 studied and carried out brine shrimp (Artemia salina) lethality bioassay to investigate the anticancer activity of chloroform and ethyl acetate extracts and the isolated compound salicylic acid (CH-2) of OT. Each sample, i.e. crude chloroform, ethyl acetate, and CH-2, was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 5 mg/mL, and a series of test solutions were taken in separate vials, i.e. 5, 10, 20, 40 and 80 μL, and in each vial, 5 mL of seawater containing 10 larvae (nauplii) was incorporated. After 1 day, each vial was checked to determine the number of larvae which were survived. The LC50 values were calculated to be 56.23, 199.53, and 31.62 μg/mL, respectively, and thus, brine shrimp lethality indicates the presence of cytotoxic action in this herb.[84,85,86]
Rashid et al. in 2002 also assessed the cytotoxic effects of these extracts, but the exact mechanism of the cytotoxic effects could not be explained by the experiment.[16]
In another experimental study, Krishnarajua et al. in 2005[87] also evaluated that the aqueous extract of OT exhibited a significant cytotoxic potential with moderate brine shrimp lethality and LC50 value was found to be 81.
Anbuselvam et al. in 2007 carried out an experiment to observe the anticancer effects of stem extracts of OT in 7,12-dimethylbenzanthracene (DMBA)-induced breast tumor in female rats model. For monitoring their antioxidant efficacy, the ethanolic extracts of stem bark was given orally at 100 mg/kg dose, and also DMBA (as an inducer) was used at a dose of 20 mg for 45 days. The findings revealed a remarkable reduction in lipid peroxidation and increased antioxidant levels with a decrease in breast tumor weight.[65]
Analgesic activity
The analgesic agents block prostaglandin biosynthesis by inhibiting cyclooxygenase enzyme. Inhibition of this enzyme centrally produces the analgesic antipyretic effect, while inhibition of this enzyme peripherally produces its anti-inflammatory effect.
Prabhavathi et al. in 2012 carried out an experiment using tail flick method and acetic acid induced the writhing response. Diclofenac sodium was employed as a standard analgesic drug. When extract was administered orally in a dose-dependent manner, it was observed that the chloroform extract of OT exhibited better dose-dependent response than the petroleum ether extract.[61]
Anti-arthritic activity
Sharma and Singh in 2013d carried out a study through the in vitro models of inhibition of protein denaturation to find the anti-arthritic activity of the ethanolic root extracts of OT. The ethanolic root extracts in various concentration with bovine serum albumin were measured for the potency. Acetylsalicylic acid was used as a standard, and finally, a significant inhibition, i.e. 70%, was observed in the case of acetylsalicylic acid while 67.22% with the ethanolic extract.[88]
Anti-inflammatory activity
Rajashekar et al. in 2006 studied and investigated the anti-inflammatory potential of OT root powder in formalin-induced edema in rats. An experiment was conducted by them in which root powder and its Ayurvedic polyherbal formulation (Avipattikar Churna) were administered orally in rats at a dose of 100 mg/kg body weight. The results showed a remarkable reduction in formalin-induced edema volume, i.e. 36.45% and 27.11%, respectively.[82]
Toxicity studies
Kumar et al. in 2006 carried out an experiment for toxicity studies of ethanolic extract of OT. When the extract was given in different groups of rat animals in a dose-dependent manner, after a particular time, the animals were observed for mortality, and it was found that there were no alterations in liver function markers such as serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase, serum bilirubin, and serum alkaline phosphatase.[66]
Another acute toxicity study was also done by Bhande et al. in 2006 in healthy albino mice for toxicity studies. In this study, healthy male/female albino rats were divided into eight different groups containing six mice in each group. Acacia suspension was administered orally at a dose of 0.5 mL in one group while root suspension of OT was administered at the dose of 10, 30, 100, 200, 400, 600, and 800 mg/kg, respectively. After then, the animals were observed at different time intervals for 1 week. Findings showed that root suspension of OT did not cause any toxic action in each group.[82]
An experiment on toxicity study was carried out by Sharma and Singh in 2012a to evaluate the acute oral toxicity using the methanolic extract of OT in mice and its LD50 value was found to 1917.66 mg/kg.[2]
CONCLUSION
In the present investigation, a set of bioactive compounds and its pharmacological activities was given on OT plant according to review literature. The preliminary phytochemical investigation reported the presence of glycosides (scopoletin, turpethinic acids [A–E]), terpenoids (lupeol, betulin), reducing sugars, alkaloids, saponins, steroids, tannins, flavonoids, coumarins, and operculinosides A–D (1–4) in different extracts of OT. The plant was found to have encouraging antimicrobial, antihepatic, antinephrotoxic, antiulcer, antidiarrheal, antidiabetic, cytotoxic, analgesic, anti-arthritic, and anti-inflammatory activities. The pharmacological actions of the plant may be due to the present of turpethin, turpethinic acid (A–E) in high quantity. The current literature review concludes that the plant OT is having significant medicinal values and safe for therapeutic treatments.
Future prospects
These studies may help in standardization, identification, and providing out further research in OT plant-based medicines which are applied in Ayurveda and modern pharmacopeia. More research is needed to isolate the various phytoconstituents present to get a clear idea of the mechanism of action of the plant. The presence of carbohydrates and reducing sugars in the plant shows the high energy content that could be utilized as a source of crude materials for pharmaceutical trades. Literature review observed that there is no clinical trials have been performed so far. Although few clinical trials have been reported safety and potency of Trvrit in some patients with rheumatoid arthritis and ascariasis, yet there is a lack of randomized, controlled clinical trial to confirm its effectiveness and safety. Such data are required to present scientific confidence to the customs use of traditional Ayurvedic drugs such as Trivrit and even be helpful for the improvement of future drugs or therapy for diseases-related rheumatoid arthritis and cancer.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kohli KR, Nipanikar SU, Kadbhane KP. A comprehensive review on Trivrit [ Operculina turpethum syn. Ipomoea turpethum] Int J Pharma Bio Sci. 2010;1:443–52. [Google Scholar]
- 2.Sharma V, Singh M. Alterations induced by N-Nitrosodimethylamine and ethanolic root extract of Operculina turpethum in serum lipid profile of male albino mice. Asian J Pharm Clin Res. 2012a;5:69–73. [Google Scholar]
- 3.Austin DF. Operculina turpethum (Convolvulaceae) as a medicinal plant in Asia. Eco Bot. 1982;36:265–9. [Google Scholar]
- 4.Vasudesan NV. Indian Medicinal Plants. Vol. 4. Chennai: Orient Longman Ltd; 1995. p. 172. [Google Scholar]
- 5.Sharma V, Singh M. Operculina turpethum as a panoramic herbal medicine: A review. Int J Pharm Sci Res. 2012b;3:21–5. [Google Scholar]
- 6.CSIR. The Wealth of India: A Dictionary of Indian Raw Materials and Industrial Products. Vol. 7. Delhi: CSIR; p. 96. [Google Scholar]
- 7.Jain NK, Saxena VK. Acta gene. Indica Chem. 1987;13:171. [Google Scholar]
- 8.Deeaph G, Malti G. Protective effect of root extract of operculina turpethum linn. Against paracetamol-induced hepatotoxicity in rats. Indian Drugs. 1994;31:294. [Google Scholar]
- 9.Nadkarni KM, Nadkarni AK, editors. Indian Materia Medica. Vol. 1. Bombay: Popular Prakasan; 2007. pp. 691–4. [Google Scholar]
- 10.Alam JM, Alam I, Sharmin AS, Rahman MM, Anisuzzaman M, Alam FM. Micropropagation and antimicrobial activity of Operculina turpethum (syn. Ipomoea turpethum), an endangered medicinal plant. Plant Omics. 2010;3:40–6. [Google Scholar]
- 11.Aswal BS, Bhakuni DS, Goel AK, Kar K, Mehrotra BN, Mukherjee KC. Screening of Indian plants for biological activity: Part X. Indian J Exp Biol. 1984;22:312–32. [PubMed] [Google Scholar]
- 12.Vaidya B. Nighantu aadarsha. Vol. 2. Varanasi, India: Chaukhamba Bharti Publishers; 2005. pp. 101–6. [Google Scholar]
- 13.Brahmanand T. Elaborated by Charaka & Drudhabala. Vol. 1. Varanasi, India: Chaukhamba Surbharti Publishers; 2008a. Charakasamhita of agnivesha; pp. 68–101. [Google Scholar]
- 14.Anantaram S. Shushrut Samhita of Maharshi Shushruta. Vol. 1. Varanasi, India: Chaukhamba Surbharti Publishers; 2008. pp. 338–47. [Google Scholar]
- 15.Sharma PV. Dravyaguna Vidnyana. Vol. 2. Varanasi, India: Chaukhamba Bharti Publishers; 2006. pp. 419–22. [Google Scholar]
- 16.Harun-or-Rashid H, Gafur MA, Golam S, Aziz AR. Antibacterial and cytotoxic activities of extracts and isolated compounds of Ipomoea turpethum. Pak J Biol Sci. 2002;5:597–9. [Google Scholar]
- 17.USDA, ARS, National Genetic Resources Program. Germplasm Resources Information Network-GRIN Taxonomy for Plants Online Database. Beltsville, Maryland: National Germplasm Resource Laboratory; [Last updated on 2002 Feb 25; Cited on 2010 May 03]. Available from: http://www.arsgrin.gov/cgibin/npgs/html/taxon.pl?25779 . [Google Scholar]
- 18.Bay Science Foundation, Inc; 1986. [Last updated on 2009 Apr 24; Cited on 2010 May 10]. Available from: http://www.zipcodezoo.com/Plants/O/Operculinaturpethum . [Google Scholar]
- 19.The International Plant Names Index. 2009. http://www.ipni.org/
- 20.Tripathi B, editor. Charaka & Drudhabala. Vol. 2. Varanasi, India: Chaukhamba Surbharti Publishers; 2008b. Charakasamhita of Agnivesha; p. 1105. [Google Scholar]
- 21.The Ayurvedic Pharmacopoeia of India, Part I. 1st ed. Vol. 3. new Delhi, India: Government of Indian Ministry of Health and Family Welfare, Department of ISM & H; 2001. pp. 213–4. 404. [Google Scholar]
- 22.Srikantha Murty KP. Bhavprakasha of bhavmishra. Vol. 1. Varanasi, India: Chaukhamba Shri Krishna Das; 2008. pp. 258–9. [Google Scholar]
- 23.Mondal A, Kabir G, Ghosh GP, Yasmin N, Alam AM, Khatun HA. Morphological variation of ten Ipomoea species of Bangladesh. Pak J Biol Sci. 2006;9:1714–9. [Google Scholar]
- 24.Ali Khan A, Khan A, Agrawal S. Gangetic khadar: One of the most threatened biomes in India. In: Rawat GS, editor. Special Habitats and Threatened Plants of India, ENVIS Bulletin: Wildlife and Protected Areas. Vol. 11. Uttrakhand: Wildlife Institute Dehradun (India); 2008. pp. 117–21. [Google Scholar]
- 25.Biswal AK, Nair MV. Threatened plants of Orissa and priority species for conservation. In: Rawat GS, editor. Special Habitats and Threatened Plants of India, ENVIS Bulletin: Wildlife and Protected Areas. Vol. 11. Uttrakhand: Wildlife Institute Dehradun (India); 2008. pp. 175–86. [Google Scholar]
- 26.Vijaya Sankar R, Ravikumar K, Goraya GS. Floristic wealth of Javvadhu Hills, Eastern Ghats, With Special Emphasis on Threatened Plants. In: Rawat GS, editor. Special Habitats and Threatened Plants of India, ENVIS Bulletin: Wildlife and Protected Areas. Vol. 11. Uttrakhand: Wildlife Institute Dehradun (India); 2008. pp. 187–93. [Google Scholar]
- 27.Molur S, Walker S. Tamilnadu: Conservation Breeding Specialist group Coimbatore (India); 1998. Report of the Conservation Assessment and Management Plan for Selected Medicinal Plant Species of Northern, North-Eastern and Central India, (BCCP-Endangered Species Project), Zoo outreach Organisation; pp. 55–6. [Google Scholar]
- 28.Mhaskar KS, Blatter E, Caius JF, Kirtikar KR, Basu’s BD. Illustrated Indian Medicinal Plants. Vol. 8. Delhi: Sri Satguru Publications; 2000. pp. 2387–90. [Google Scholar]
- 29.Rastogi R, Mehrotra BN, Sinha S, Pant P, Sheth R. Compendium of Indian Medicinal Plants. Vol. 2. New Delhi, India: CDRI Lucknow and National Institute of Science Communication; 2006. p. 499. [Google Scholar]
- 30.Rastogi R, Mehrotra BN, Sinha S, Shrivastava M, Bhushan B. Compendium of Indian Medicinal Plants. Vol. 4. New Delhi, India: CDRI Lucknow & National Institute of Science Communication; 2002. p. 513. [Google Scholar]
- 31.Yoganarasimhan SN. Medicinal Plants of India. Bangalore: Self Publication; 2005. p. 41. [Google Scholar]
- 32.Shah CS, Qadry JS, Krishnamurthy TN. Sugars and coumarins in black-turpeth (Ipomea turpethum) Indian J Pharm. 1972;34:126. [Google Scholar]
- 33.Ding W, Zeng F, Xu L, Chen Y, Wang Y, Wei X. Bioactive dammarane-type saponins from Operculina turpethum. J Nat Prod. 2011;74:1868–74. doi: 10.1021/np200274m. [DOI] [PubMed] [Google Scholar]
- 34.Shareef H, Mahmood S, Farrukh U, Ahmed A, Rizwani GH. In vitro antimicrobial and phytochemical activity of Operculina turpethum L. inventi impact. Ethnopharmacol. 2010;1:50–3. [Google Scholar]
- 35.Ramkumar KM, Manjula C, Sankar L, Suriyanarayanan S, Rajaguru P. Potential in vitro antioxidant and protective effects of Gymnema montanum H on alloxan induced oxidative damage in pancreatic β-cells, HIT-T15. Food Chem Toxicol. 2009;47:2246–56. doi: 10.1016/j.fct.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Jalaj AV, Madhavan RP. Pharmacognostic studies on leaf of Operculina turpethum (L.). Silva Manso. Int J Adv Res. 2014;2:585–90. [Google Scholar]
- 37.Veena S, Manu S. Comparative analysis of the phytochemicals present in different extracts of Operculina turpethum. Int J Drug Dev Res. 2013a;5:244–50. [Google Scholar]
- 38.Aboaba OO, Efuwape BM. Antibacterial properties of some Nigerian species. Biol Res Comm. 2001;13:183–8. [Google Scholar]
- 39.Jalaj AV, Madhavan RP. Identification and quantification of phenolic compounds from O. turpethum (L.) Silva Manso leaf by HPLC method. IJPSR. 2016;7:1656–61. [Google Scholar]
- 40.Malik CP, Singh MB. Plant Enzymology and Histo-Enzymology. New Delhi: Kalyani Publishers; 1980. p. 286. [Google Scholar]
- 41.El-Far MM, Taie AA. Antioxidant activities: Total anthocyanins, phenolics and flavonoids contents of some sweet potato genotypes under stress of different concentrations of sucrose and sorbitol. Australian J Basic Appl Sci. 2009;3:3609–16. [Google Scholar]
- 42.Huang WY, Cai YZ, Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr Cancer. 2010;62:1–20. doi: 10.1080/01635580903191585. [DOI] [PubMed] [Google Scholar]
- 43.Attele AS, Wu JA, Yuan C. Analgesic effects of different auc point stimulation frequencies in humans. Biochem Pharmacol. 1999;58:1685–93. [Google Scholar]
- 44.Finar IL. Stereochemistry and the Chemistry of Natural Products. 5th ed. Vol. 2. UK: Longman Group; 1989. Chemistry; pp. 517–605. [Google Scholar]
- 45.Trease GE, Evans MD. A Text Book of Pharmacognosy. 13th ed. London: Baillier, Tindal and Caussel; 1989. pp. 144–8. [Google Scholar]
- 46.Gopish KV, Kannabiran K. Antimicrobial activity of saponin fractions of the leaves Gymnema sylvestre and Eclipta prostrata. World J Microbiol Biotechnol. 2008;24:2737–40. [Google Scholar]
- 47.Arif A, Howlader Sariful I, Dey Shubhra K, Hira A, Hemayet H, Nasir Uddin MM. Phytochemical screening and antibacterial activity of different fractions of Operculina turpethum root and leaf. Am J Sci Ind Res. 2013;4:167–72. [Google Scholar]
- 48.Doss A, Muhamed Mubarack H, Dhanabalan R. Antibacterial activity of tannins from Solanum trilobatum Linn. leaves. Indian J Sci Tech. 2009;2:41–3. [Google Scholar]
- 49.Doss A, Vijaya Santhi M, Parivuguna V, Venkataswamy R. Antimicrobial effects of the flavonoid fractions of Mimosa pudica L. leaves. J Pharm Res. 2011a;4:1438–9. [Google Scholar]
- 50.Doss A, Parivuguna V, Vijaya Santhi M, Sruthi S. Antibacterial and preliminary phytochemical analysis of Medicago sativa L. against some microbial pathogens. Indian J Sci Tech. 2011b;4:550–2. [Google Scholar]
- 51.Lamson DW, Brignall MS. Antioxidants and cancer, Part 3: Quercetin. Altern Med Rev. 2000;5:196–208. [PubMed] [Google Scholar]
- 52.Rani SA, Murty SU. Antifungal potential of flower head extract of Spilanthes acmella Linn. Afr J Biomed Res. 2006;9:67–8. [Google Scholar]
- 53.Verpoorte R. Antimicrobially active alkaloids. In: Robert MF, Wink M, editors. Alkaloids: Biochemistry, Ecology and Medicinal Applications. Australia: Springer Publication; 1998. pp. 397–426. [Google Scholar]
- 54.Slobodníková L, Kost’álová D, Labudová D, Kotulová D, Kettmann V. Antimicrobial activity of Mahonia aquifolium crude extract and its major isolated alkaloids. Phytother Res. 2004;18:674–6. doi: 10.1002/ptr.1517. [DOI] [PubMed] [Google Scholar]
- 55.Duraiswamy B, Sagar KM, Subhashini V, Dhanraj SA, Suresh B. Studies on the antimicrobial potential of Mahonia leschenaultia Takeda root and root bark. Indian J Pharm Sci. 2006;68:389–91. [Google Scholar]
- 56.Li AR, Zhu Y, Li XN, Tian XJ. Antimicrobial activity of four species of Berberidaceae. Fitoterapia. 2007;78:379–81. doi: 10.1016/j.fitote.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Harborne JB. Phytochemicals Methods. London: Hapman and Hall Ltd; 1973. pp. 49–188. [Google Scholar]
- 58.Basak UC, Mohapatra M. Comparative assessment of coumarin content in root barks and seeds of Operculina turpethum. A vulnerable medicinal plant of Odisha, India. IJIPSR. 2015;3:1330–8. [Google Scholar]
- 59.Kostova I, Bhatia S, Grigorov P, Balkansky S, Parmar VS, Prasad AK, et al. Coumarins as antioxidants. Curr Med Chem. 2011;18:3929–51. doi: 10.2174/092986711803414395. [DOI] [PubMed] [Google Scholar]
- 60.Kumari S, Meena AK, Shukla VJ, Ota S, Rao MM. Quality assessment of Ipomoea species plants by HPTLC. J Anal Chem. 2010;1:1–8. [Google Scholar]
- 61.Prabhavathi NB, Kowsalya B, Kumar SR, Sravani BJ, Sri GD, Sakila A, et al. Analgesic activity of different solvent extract of Operculina turpethum by using swiss albino mice. Asian J Pharm Clin Res. 2012;5:215–8. [Google Scholar]
- 62.Sharma V, Singh M. Non-opiodergic like mechanism for antinociceptive analgesic and antipyretic activity of ethanolic root extract of Operculina turpethum in swiss albino mice. Int J Pharm Bio Sci. 2013b;4:104–2. [Google Scholar]
- 63.Venugopala KN, Rashmi V, Odhav B. Review on natural Coumarin lead compounds for their pharmacological activity. Biomed Res Int. 2013;2013:1–14. doi: 10.1155/2013/963248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kirtikar KR, Basu DB. Blatter E, Caius JF, Mahaskr KS. Indian Medicinal Plant. Vol. 3. Dehradun: India; 1987. pp. 1730–32. [Google Scholar]
- 65.Anbuselvam C, Vijayavel K, Balasubramanian MP. Protective effect of Operculina turpethum against 7,12-dimethyl benz (a) anthracene induced oxidative stress with reference to breast cancer in experimental rats. Chem Biol Interact. 2007;168:229–36. doi: 10.1016/j.cbi.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 66.Kumar SV, Sujatha C, Syamala J, Nagasudha B, Mishra SH. Protective effect of root extract of Operculina turpethum Linn. against paracetamol-induced hepatotoxicity in rats. Indian J Pharm Sci. 2006;68:32–5. [Google Scholar]
- 67.Pulipaka S, Challa SR, Pingili RB. Comparative antidiabetic activity of Operculina turpethum stem and root against healthy and streptozotocin induced diabetic rats. Int Curr Pharma J. 2012;1:272–8. [Google Scholar]
- 68.Sharma V, Singh M. Attenuation of N-nitrosodimethylamine induced hepatotoxicity by Operculina turpethum in Swiss Albino mice. Iran J Basic Med Sci. 2014;17:73–80. [PMC free article] [PubMed] [Google Scholar]
- 69.Sharma V, Singh M. Ameliorative effects of Operculina turpethum and its isolated stigma-5,22dien-3-o-β-D-glucopyranoside on the hematological parameters of male mice exposed to N-nitrosodimethylamine, a potent carcinogen. Toxicol Int. 2014b;21:29–36. doi: 10.4103/0971-6580.128789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aoyama Y, Katayama T, Yamamoto M, Tanaka H, Kon K. A new antitumor antibiotic product, demethylchartreusin. Isolation and biological activities. J Antibiot (Tokyo) 1992;45:875–8. doi: 10.7164/antibiotics.45.875. [DOI] [PubMed] [Google Scholar]
- 71.Borges F, Roleira F, Milhazes N, Santana L, Uriarte E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr Med Chem. 2005;12:887–916. doi: 10.2174/0929867053507315. [DOI] [PubMed] [Google Scholar]
- 72.García-Argáez AN, Ramírez Apan TO, Parra Delgado H, Velázquez G, Martínez-Vázquez M. Anti-inflammatory activity of coumarins from Decatropis bicolor on TPA ear mice model. Planta Med. 2000;66:279–81. doi: 10.1055/s-2000-14894. [DOI] [PubMed] [Google Scholar]
- 73.Sardari S, Mori Y, Horita K, Micetich RG, Nishibe S, Daneshtalab M. Synthesis and antifungal activity of coumarins and angular furanocoumarins. Bioorg Med Chem. 1999;7:1933–40. doi: 10.1016/s0968-0896(99)00138-8. [DOI] [PubMed] [Google Scholar]
- 74.Kwon YS, Kobayashi A, Kajiyama S, Kawazu K, Kanzaki H, Kim CM. Antimicrobial constituents of Angelica dahurica roots. Phytochemistry. 1997;44:887–9. doi: 10.1016/s0031-9422(96)00634-6. [DOI] [PubMed] [Google Scholar]
- 75.Fujioka T, Furumi K, Fujii H, Okabe H, Mihashi K, Nakano Y, et al. Antiproliferative constituents from umbelliferae plants. V. A new furanocoumarin and falcarindiol furanocoumarin ethers from the root of Angelica japonica. Chem Pharm Bull (Tokyo) 1999;47:96–100. doi: 10.1248/cpb.47.96. [DOI] [PubMed] [Google Scholar]
- 76.Mirunalini S, Krishnaveni M. Coumarin: A plant derived polyphenol with wide biomedical applications. Int J PharmTech Res. 2011;3:1693–6. [Google Scholar]
- 77.Sharma V, Singh M. Isolation and characterization of stigma-5,22dien-3-O-β-D-glucopyranoside from the ethanolic root extract of Operculina turpethum. Int J Adv Res. 2013c;1:303–12. [Google Scholar]
- 78.Srivastava OP. Dewn BN, Srinath R, editors. Techniques for evaluation of antimicrobial properties of natural product. The Use of Pharmacological Techniques for Evaluation of Natural Product. 75:1984. [Google Scholar]
- 79.Reiner R. Antibiotics: An Introduction. Switzerland: Roche Scientific Service; 1982. Detection of antibiotic activity; pp. 21–5. [Google Scholar]
- 80.Prakash VB, Mukherjee A. Hepato-protective effect of an ayurvedic formulation prak-20 in ccl4 induced toxicity in rats: Results of three studies. Int J Pharm Clin Res. 2010;2:23–7. [Google Scholar]
- 81.Ignatius V, Narayanan M, Subramanian V, Periyasamy BM. Antiulcer activity of indigenous plant Operculina turpethum Linn. Evid Based Complement Alternat Med. 2013;2013:272134. doi: 10.1155/2013/272134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhande RM, Laakshmayya Kumar P, Mahurkar NK, Ramachandra Setty S. Pharmacological screening of root of Operculina turpethum and its formulations. Acta Pharm Sci. 2006;48:11–7. [Google Scholar]
- 83.Shareef H, Rizwani GH, Mandukhail SR, Watanabe N, Gilani AH. Studies on antidiarrhoeal, antispasmodic and bronchodilator activities of Operculina turpethum Linn. BMC Complement Altern Med. 2014;14:479. doi: 10.1186/1472-6882-14-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–4. [PubMed] [Google Scholar]
- 85.Persoone G. Artemia Salina Vois. Belgium: Universa Press, Witteren; 1980. Proceeding of the International Symposium on brine Shrimp; pp. 1–3. [Google Scholar]
- 86.Mclaughlin JL, Anderson JE. Proceeding NIH Workshop. Bioassay for Discovery of Antitumor and Antiviral Agents from Natural Sources. Bethesda: 1988. Brine Shrimp and Crown Gal Tumors: Simple Bioassay for the Discovery of Plant Antitumor Agent; p. 22. [Google Scholar]
- 87.Krishnarajua AV, Raoa VN, Sundararajua D, Vanisreeb M, Tsayb HS, Subbarajua GV. Assessment of bioactivity of Indian medicinal plants using brine shrimp (Artemia salina) lethality assay. Int J Appl Sci Eng. 2005;3:125–34. [Google Scholar]
- 88.Sharma V, Singh M. In vitro antiarthritic and hemolysis preventive: Membrane stabilizing efficacy of ethanolic root extract of Operculina turpethum. World J Pharm Pharm Sci. 2013d;2:302–12. [Google Scholar]


