Figure 6.
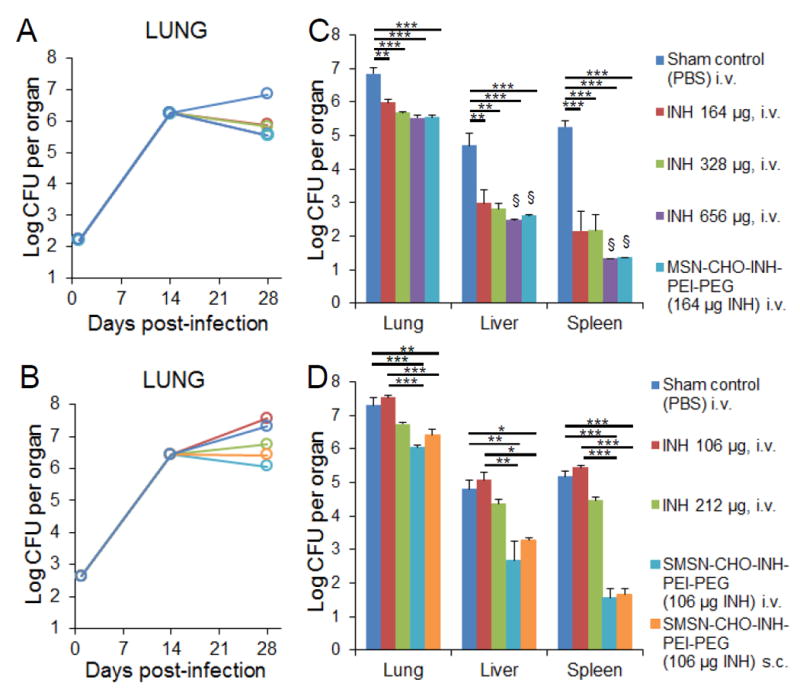
In vivo efficacy of MSN-CHO-INH-PEI-PEG and SMSN-CHO-INH-PEI-PEG. Mice were infected with A) 250 and B) 500 live M. tuberculosis bacilli by aerosol. Bacterial burdens in the lung were monitored throughout the course of infection. The effect of C) MSN-CHO-INH-PEI-PEG and D) SMSN-CHO-INH-PEI-PEG treatments on M. tuberculosis burden in lung, liver, and spleen was determined by assaying M. tuberculosis CFU three days after the final treatment. The equivalent amount of free INH for each type of nanoparticle is shown in parenthesis. Statistics were analyzed using one-way ANOVA with Bonferroni post-test correction. *p< 0.1, **p< 0.01, ***p< 0.001. Error bars represent standard errors with 3 mice per group. §Organ bacterial CFU below the experimental limit of detection.
