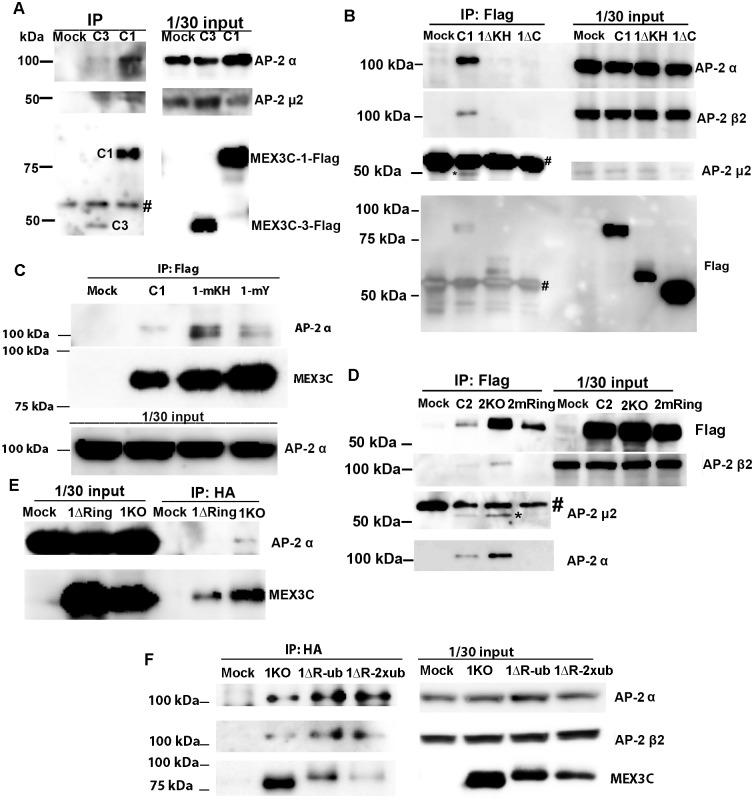
Fig 2. Mapping the MEX3C regions mediating MEX3C/AP-2 interaction.
A. MEX3C-1 and MEX3C-3 pull down endogenous AP-2 subunits. Transiently expressed MEX3C-1 and MEX3C-3 were Flag-tagged. C1 and C3 indicate MEX3C-1-Flag and MEX3C-3-Flag respectively. # indicates the IgG band. B. Deleting the central region or the C-terminal 249AA of MEX3C-1 abolished MEX3C/AP-2 interaction. MEX3C-1 (C1 in Figure), MEX3C-1-ΔKH (1ΔKH) and MEX3C-1-ΔC (1ΔC) were Flag-tagged and detected with anti-Flag antibody. MEX3C-1-ΔC appeared at the same position as the IgG heavy chain. # and * indicate the IgG and the AP-2 μ2 bands, respectively. C. Mutating the YXXΦ-like motifs (lane 1-mY) or the amino acids necessary for RNA binding (lane 1-mKH) did not affect MEX3C/AP-2 interaction. Transiently expressed MEX3C-1, MEX3C-1-mY, and MEX3C-1-mKH were Flag-tagged and immunoprecipitated by anti-Flag antibody to detect co-immunoprecipitated AP-2 subunits. The AP-2 α subunit pulled down by both mutants contained a smaller band in addition to the normally observed large band. D. The ring finger domain of MEX3C is necessary for MEX3C/AP-2 interaction. MEX3C-2 (C2 in Figure), MEX3C-2-KO (all lysine residues were mutated to arginine, lane “2KO”), and MEX3C-2-mRing (the C3HC4 motif in the MEX3C-2 ring finger domain was mutated to A3NC4, lane “2mRing”) were Flag-tagged. # indicates the IgG band and * indicates the AP-2 μ2 band. E. Deleting the ring finger domain (C-terminal 53 AA) of MEX3C-1 abolished MEX3C/AP-2 interaction. 1ΔRing: MEX3C-1-KO-ΔRing, all lysine residues of MEX3C-1 were mutated to arginine and the ring finger domain was deleted. 1KO: All lysine residues in MEX3C-1 were changed to arginine. Both mutants were HA-tagged and immunoprecipitated by anti-HA antibody to detect AP-2 subunits. F. Addition of ubiquitin chain(s) to MEX3C-1-KO-ΔRing restored MEX3C/AP-2 interaction. 1ΔR-ub: MEX3C-1-KO-ΔRing-UbKO, MEX3C-1-KO-ΔRing fused to one ubiquitin chain whose lysine residues were all mutated to arginine; 1ΔR-2xub: MEX3C-1-KO-ΔRing-2xUbKO, MEX3C-1-KO-ΔRing fused to two ubiquitin chains whose lysine residues were all mutated to arginine. All three mutants were HA-tagged. For A~F, “Mock” indicates vector-DNA transfected cells.