Abstract
Amadumbe, known as taro is a traditional crop mainly grown for subsistence in Southern Africa. In this study, chemical composition and functional properties of nine amadumbe genotypes grown at two distinct locations were investigated. Carbohydrate contents (73–81%) of amadumbe genotypes were substantially high and varied with growth location. Protein contents ranged from 8–12% and fat was very low (less 1%) in all genotypes. Major minerals in flours were K, P, Mg and Ca, but these were present at varying levels depending on growth locations. Amadumbe flours showed slightly low mucilage contents (6–9%) across genotypes. However, genotypes with higher mucilage contents generally had higher water absorption capacities irrespective of growth locations. Genotype and growth location significantly affected the pasting properties of amadumbe flours. Peak viscosities varied between 83–242 RVU among genotypes. The pasting temperature of the genotypes were fairly high 87–94 °C across genotypes. This study data suggests that differences in environmental temperatures and amounts of rain falls received at growth location during the growing season could be some of the factors responsible for the variations in flour composition and consequently their functionality. Findings from this study are important for future improvement programme and for food application of amadumbe flour.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2816-0) contains supplementary material, which is available to authorized users.
Keywords: Amadumbe genotypes, Growth location, Flour, Composition, Functionality
Introduction
Amadumbe (Colocasia esculenta), also known as taro are grown for their edible corms in the tropics and subtropical parts of the world (Huang et al. 2010). In South Africa, amadumbe is a traditional tuber crop grown for subsistence. The corms are normally boiled, fried or made into a mash to create a variety of dishes. Amadumbe is rich in carbohydrates (75–90%), the bulk of it being starch (approx. 80–96%) (Aboubakar et al. 2008; Aprianita et al. 2009; Naidoo et al. 2015). The high resistant starch content (approx. 60%) (Naidoo et al. 2015) and mucilage (7–10%) of amadumbe suggest that amadumbe corms may be important for digestive health (Guevara-Arauza et al. 2012; Hong and Nip 1990). Furthermore, amadumbe corms are fairly good sources of vitamins and minerals such as K, Zn, Mg and Mn, which play significant roles in the metabolic process (Mergedus et al. 2015). As such, amadumbe flour can be used as ingredient in the food formulations for health-conscious individuals, i.e. obese or diabetics (Liu et al. 2006).
Due to their high moisture content (approx. 90%), amadumbe tubers are highly perishable. Hence, they must be processed into stable products such as flours (Falade and Okafor 2015). The use of flours in the food industry as food ingredient is primarily governed by their functional and physicochemical properties. Flour composition may be influenced by growth location, which may impact functionality and application. According to Tester and Karkalas (2001), environmental factors such as growth temperature may influence starch composition (e.g. amylose content), which in turn can modify the properties of flour. For instance, Tattiyakul et al. (2006) studied the composition and functional properties of taro varieties grown at four different locations in Thailand. The protein contents of flour (0.9–1.7 g/100 g flour) varied with growth location indicating an environmental influence on the composition of taro flours. Also, the swelling power (11.3–15.9 g/g) and solubility (0.08–0.13 g/g) of the flours have been found to differ with growth location (Tattiyakul et al. 2006). Besides these environmental factors, genotypic differences may also influence flour functionality such as pasting properties. Falade and Okafor (2015) found significant differences in the peak viscosities (97.3–201.2 RVU) of taro genotypes grown in the same location.
In Southern Africa, amadumbe is regarded as traditional crop and its utilisation remains largely domestic. However, amadumbe flour has the potential for industrial application, since it has some beneficial physiological effect as stated above. Previous studies on amadumbe investigated water use and drought resistance of locally grown varieties in South Africa (Mabhaudhi et al. 2013). These studies revealed significant variations among amadumbe landraces grown under varying environmental conditions. Under different irrigation treatments, amadumbe corm mass reportedly reduced with a decrease in soil water availability (Mabhaudhi et al. 2013). However, the effort to promote the utilisation of amadumbe in South Africa has focused on breeding of locally grown varieties, largely on the agro-morphological and molecular markers. The integration of breeding for yield and yield-related traits, as well as physicochemical properties, is important for food and nutritional security. Although breeding data was encouraging, breeding can interfere with the good inherent properties of amadumbe flours. Hence, in this study, the composition and functional properties of flours extracted from amadumbe genotypes grown under different environmental conditions were investigated.
Materials and methods
Nine genotypes were obtained from the Agricultural Research Council-Vegetable and Ornamental Plant Institute, Pretoria, South Africa. The genotypes were grown at Roodeplaat research farm and Umbumbulu farmers’ field. The genotypes were evaluated for their agro-morphological characteristics. The altitude of Umbumbulu and Roodeplaat is 597 m and 1168 m above sea level, respectively. The locations receive an annual rainfall of 828 mm and 514 mm for Umbumbulu and Roodeplaat, respectively. The average temperatures for Roodeplaat and Umbumbulu were 19 and 24 °C, respectively for the cropping season (Sept/2014- May/2015) (Supplementary data).
Flour preparation Freshly harvested amadumbe corms were washed, peeled, and sliced into a thickness of three mm. Peeled corms were dried at 50 °C for 48 h in a hot air oven (D-37520, Thermo Fisher Scientific, Germany). Dried slices were then milled into flour using a warring blender (Model: 8010S, Torrington, USA) and sieved (screen size: 180 mm) to obtain fine flours, which were then stored at 4 °C prior to analysis.
Proximate compositions Moisture, fat, ash and protein content of amadumbe flours was determined using (AOAC 2000) methods. Protein content (N× 6.25) was determined by Kjeldahl method (6.25 ×N) and total carbohydrate was calculated by difference.
Mineral profile determination Phosphorous content of flours, as a percentage of sample weight (w/w), was determined following established spectrophotometric AOAC (2000) method while minerals (Ca, Na, Mg, K, Mn, Mg, Fe, Zn, Cu) content, as a percentage of sample weight (w/w), was analysed using an atomic absorption (AA) spectrophotometer.
Water and oil absorption capacity Water absorption capacity was done according to the method followed by Falade and Okafor (2015).
Swelling power and Solubility index Swelling power and solubility index were determined following methods described by Gebresamuel and Gebre-Mariam (2012).
Pasting property The pasting properties of isolated amadumbe flours were determined using the Rapid Visco Analyser (RAV-4, Newport, Scientific, Warriewood, Australia) following established method followed by Oyeyinka et al. (2016). Parameters recorded were pasting temperatures, peak viscosity, trough viscosity (min viscosity @ 95 °C), final viscosity (viscosity @50 °C), breakdown viscosity (peak-trough viscosity) and setback viscosity (final viscosity).
Statistical analysis The data reported in all the tables are average values of triplicate determinations. The data were analysed using two-way analysis of variance (ANOVA) and the means were compared using the Fisher Least significant difference (LSD) test (p < 0.05). The variations observed in the functional and pasting properties of flours obtained from different growth locations and genotypes were examined by principal component analysis (PCA).
Results and discussion
Proximate composition of amadumbe flours Proximate composition of flours isolated from amadumbe grown in Roodeplaat (R) and Umbumbulu (U) varied significantly (Table 1). Amadumbe flours from genotypes grown in both locations showed high carbohydrate content ranging between 72–80% while fats, protein, and ash were lower (Table 1). The carbohydrate content was generally low compared to previously reported data on taro flours (Aboubakar et al. 2008; Kaur et al. 2013). However, between the two locations, amadumbe flour obtained from genotypes grown in Umbumbulu showed slightly higher carbohydrate content approx. 77% when compared to the same genotypes grown at Roodeplaat (approx. 74%). Higher annual rainfall received in Umbumbulu (828 mm) could have caused higher carbohydrate content due to increased enzymatic activities in starch biosynthesis resulting in accumulation of starch granules compared to lower rainfall received in Roodeplaat (514 mm) which could have resulted in lower CHO content (Zhong-Min et al. 2008). Soil water deficit has been reported to cause reduced enzymatic activities, resulting in lower CHO content due to less accumulation of starch granules (Zhong-Min et al. 2008). Comparable results have been reported by Thitisaksakul et al. (2012), who found out that soil water deficit resulted in decrease of cereal starch accumulation by 40% which consequently result in reduced CHO content. Moreover, the differences in the carbohydrate values may be due to variation in soil composition, agronomic practices in which these amadumbe were grown or possibly genotypic differences. Furthermore, ash content which represents the mineral component and protein content varied from approx. 4–8 and 8–12%, respectively. These values were generally high compared to those reported in literature for taro flours (Aboubakar et al. 2008; Kaur et al. 2013; Naidoo et al. 2015). This difference in composition could be attributed to differences in soils nutrients at two growth locations.
Table 1.
Proximate composition of flour isolated from amadumbe genotypes grown in different locations
| Locations | Parameters | Moisture (%) | Protein (%) | Ash (%) | ADF (%) | NDF (%) | Fats (%) | CHO (%) |
|---|---|---|---|---|---|---|---|---|
| Genotypes | ||||||||
| Roodeplaat | G1 | 10.4b | 8.92d | 6.81b | 5.91c | 50.9a | 0.77b | 73.1c |
| G2 | 8.69e | 7.68f | 6.21c | 6.88b | 50.7a | 0.50f | 76.9b | |
| G3 | 9.28c | 8.09e | 6.80b | 5.39 cd | 40.9b | 0.66d | 75.2bc | |
| G9 | 8.32d | 9.77b | 6.48bc | 7.13a | 29.2d | 0.28 g | 75.2bc | |
| G20 | 11.1a | 9.31c | 6.58bc | 5.24 cd | 51.2a | 0.71c | 72.3c | |
| G21 | 8.53d | 9.65bc | 7.61a | 6.76bc | 41.8b | 0.56e | 73.7c | |
| G22 | 10.2bc | 6.87 g | 6.50bc | 5.18d | 49.0ab | 0.84a | 80.6a | |
| G26 | 8.68d | 8.69de | 6.31c | 6.66bc | 26.6d | 0.62d | 75.7bc | |
| G29 | 6.36e | 10.3a | 6.63bc | 6.15c | 33.7c | 0.82ab | 75.9bc | |
| Max | 11.1 | 10.3 | 7.61 | 7.13 | 51.2 | 0.84 | 80.6 | |
| Min | 6.36 | 6.87 | 6.21 | 5.18 | 26.6 | 0.28 | 72.3 | |
| C.V | 0.27 | 0.42 | 0.86 | 0.38 | 0.12 | 6.94 | 0.05 | |
| Umbumbulu | G1 | 7.13 cd | 9.83c | 5.04ab | 4.94c | 52.2a | 0.63c | 77.4a |
| G2 | 4.42f | 9.75c | 5.04ab | 5.66b | 33.8c | 0.61c | 73.2ab | |
| G3 | 7.34c | 7.97de | 4.66bc | 5.28bc | 27.1d | 0.42e | 75.6a | |
| G9 | 6.11e | 8.35d | 5.13a | 6.28a | 30.6 cd | 0.61c | 77.8a | |
| G20 | 6.51d | 12.0a | 4.34c | 5.62b | 50.3ab | 0.55d | 76.6a | |
| G21 | 8.69b | 8.35d | 4.74bc | 4.69c | 46.4b | 0.76b | 75.5a | |
| G22 | 6.02e | 7.19e | 4.69bc | 5.60b | 46.8b | 0.83a | 75.3a | |
| G26 | 7.31c | 10.8b | 4.47bc | 4.55c | 52.2a | 0.72bc | 76.7a | |
| G29 | 9.29a | 11.4ab | 4.97b | 6.00ab | 43.1bc | 0.47e | 73.8ab | |
| Max | 9.29 | 12.0 | 5.13 | 6.28 | 52.2 | 0.83 | 77.8 | |
| Min | 4.42 | 7.19 | 4.34 | 4.55 | 27.1 | 0.42 | 73.2 | |
| C.V | 0.84 | 0.21 | 0.44 | 0.33 | 0.06 | 3.23 | 0.03 | |
Mean ± SD Mean values with different letters in column are significantly different (p<0.05), Max maximum value, Min minimum value, C.V coefficient of value, G genotype, G with a number is the identification code of the genotype (Gn), ADF acid detergent fiber, NDF Neutral detergent fiber, CHO Carbohydrates
Mineral composition
The mineral composition of flours isolated from amadumbe genotypes grown in Roodeplaat and Umbumbulu varied significantly (p < 0.05) (Table 2). Ca, Mg, K, Na and P were present in high amount in flours isolated from amadumbe grown in Roodeplaat compared to those obtained from Umbumbulu. Amadumbe flours from two locations showed substantially high levels of K, approx. 2457 mg/100 g and 1952 mg/100 g for Roodeplaat and Umbumbulu, respectively. Potassium (K) is a vital nutrient which plays a pivotal role in human health such as relief from stroke, blood pressure, heart and kidney disorders (Ndabikunze et al. 2011). The macromineral values of flours from this study were fairly high when compared to reports in literature for taro flours (Aboubakar et al. 2008; Polycarp et al. 2012). The differences in Ca and Mg of amadumbe flours can be attributed to differences in growth location. For instance, according to Ndabikunze et al. (2011), taro grown in swampy areas exhibited higher Ca and Mg content of (110 and 90 mg/100 g) than those grown in dry lands (69 and 84 mg/100 g). Further, Zn, Cu, Mn and Fe also varied significantly (p<0.05) with genotype and growth location. Flours isolated from amadumbe genotypes from Roodeplaat showed a higher amount of Zn (approx. 137 ppm), Cu (12 ppm) and Fe (31 ppm) when compared to those obtained from Umbumbulu. The difference in these mineral elements can be attributed to variations in soil nutritional composition. However, these micronutrient values are much lower compared to those reported in the literature, which are fairly high (Ndabikunze et al. 2011).
Table 2.
Mineral composition of flour extracted from amadumbe genotypes grown in different locations
| Locations | Parameters | Ca g/ 100 g | Mg | K | Na | P | Zn mg/kg | Cu | Mn | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Genotypes | ||||||||||
| Roodeplaat | G1 | 70.0 cd | 250a | 2570b | 20.0bc | 770a | 149ab | 13.0b | 5.50bc | 27.5b |
| G2 | 80.0c | 240a | 2260e | 50.0a | 730ab | 133c | 12.5b | 7.00b | 26.5b | |
| G3 | 80.0c | 240a | 2560b | 30.0b | 770a | 135c | 13.5b | 6.50b | 35.0a | |
| G9 | 120a | 200a | 2360d | 40.0ab | 690b | 151ab | 12.5b | 8.50a | 34.5a | |
| G20 | 50.0d | 220a | 2410 cd | 40.0ab | 750a | 121d | 12.5b | 5.00c | 26.5b | |
| G21 | 80.0c | 200a | 2870a | 50.0a | 740a | 113e | 9.00b | 5.50bc | 36.5a | |
| G22 | 80.0c | 220a | 2460c | 20.0bc | 670b | 138c | 11.5b | 5.00c | 32.5a | |
| G26 | 120a | 200a | 2320d | 40.0ab | 610bc | 144b | 13.0b | 7.00b | 31.5ab | |
| G29 | 100b | 240a | 2310d | 40.0ab | 670b | 155a | 25.0a | 9.00a | 32.5a | |
| Max | 120 | 250 | 2870 | 50.0 | 770 | 155 | 25.0 | 9.00 | 36.5 | |
| Min | 50.0 | 200 | 2260 | 20.0 | 610 | 112.50 | 9.00 | 5.00 | 26.5 | |
| C.V | 0.02 | 0.02 | 0.00 | 0.09 | 0.00 | 0.07 | 5.88 | 8.92 | 0.37 | |
| Umbumbulu | G1 | 30.0b | 120a | 2060ab | 20.0a | 180b | 25.5b | 6.50b | 14.0c | 21.5c |
| G2 | 40.0ab | 130a | 2040b | 20.0a | 170b | 25.0b | 6.50b | 11.5 cd | 16.5d | |
| G3 | 30.0b | 120a | 1910c | 10.0a | 160bc | 34.0a | 7.00b | 14.0c | 28.0b | |
| G9 | 50.0ab | 120a | 2100a | 10.0a | 200a | 26.5b | 7.50ab | 22.5a | 20.0c | |
| G20 | 40.0ab | 140a | 1720d | 20.0a | 170bc | 24.0b | 6.50b | 8.50d | 19.5c | |
| G21 | 60.0a | 120a | 1920c | 20.0a | 180b | 22.5bc | 4.00c | 13.5c | 20.0c | |
| G22 | 60.0a | 120a | 1920c | 20.0a | 190ab | 33.0a | 8.00ab | 18.0b | 24.0bc | |
| G26 | 30.0b | 140a | 1830 cd | 10.0a | 140c | 31.5ab | 6.50b | 17.5b | 27.0b | |
| G29 | 40.0ab | 120a | 2070ab | 20.0a | 190ab | 25.5b | 9.50a | 14.0c | 36.0a | |
| Max | 60.0 | 140 | 2100 | 20.0 | 200 | 34.00 | 9.50 | 22.5 | 36.0 | |
| Min | 30.0 | 120 | 1720 | 10.0 | 140 | 22.50 | 4.00 | 8.50 | 16.5 | |
| C.V | 0.04 | 0.01 | 0 | 0.16 | 0.08 | 0.42 | 8.74 | 4.02 | 1.53 | |
Mean ± SD Mean values with different letters in column are significantly different (p<0.05), Max maximum value, Min minimum value, C.V coefficient of value, G genotype, G with a number is the identification code of the genotype (Gn)
Crude Mucilage of amadumbe The crude mucilage content of flours was significantly affected by genotype and growing location. Generally, the crude mucilage was low and ranged between 6–9% for amadumbe genotypes grown at both locations (Table 3). On average, amadumbe genotypes grown in Roodeplaat with lower growth temperature (19 °C) showed slightly higher mucilage content than their counterparts grown in Umbumbulu with elevated growth temperature (24 °C). Slightly higher values of mucilage for taro (approx. 10%) have been previously reported (Hong and Nip 1990). Differences in mucilage content can be attributed to environmental plant stress response during growth (Jiang and Ramsden 1999). Some authors have postulated that elevated growth temperatures could result in decrease or increase of mucilage content subject to genotype response to stress (Aprianita 2010), and this was clearly seen in this study. Mucilage can be used as functional ingredient (soluble fibre) or as a thickener/stabiliser since it exhibits unique rheological properties.
Table 3.
Crude mucilage content for flour extracted from amadumbe genotypes grown in different locations
| Parameters | Crude mucilage content (%) | |
|---|---|---|
| Locations | Roodeplaat | Umbumbulu |
| Genotypes | ||
| G1 | 7.26bc | 6.98b |
| G2 | 7.81b | 7.10b |
| G3 | 6.63 cd | 7.46ab |
| G9 | 7.34bc | 6.87b |
| G20 | 7.06c | 6.79b |
| G21 | 7.64b | 5.80c |
| G22 | 8.82a | 8.53a |
| G26 | 7.16bc | 6.60b |
| G29 | 6.45d | 6.51b |
| Max | 8.82 | 8.53 |
| Min | 6.45 | 5.80 |
| C.V | 1.45 | 7.41 |
Mean ± SD Mean values with different letters in column are significantly different (p<0.05), Max maximum value, Min minimum value, C.V coefficient of value, G genotype, G with a number is the identification code of the genotype (Gn)
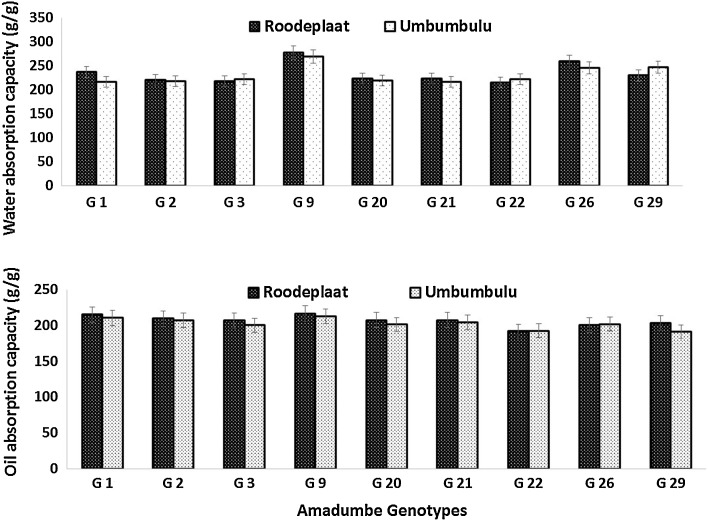
Water absorption Water absorption capacity (WAC) of amadumbe flours was almost similar across all genotypes grown in both Roodeplaat (R) and Umbumbulu (U). Only slight differences were observed across all genotypes (Fig. 1). However, genotypes, G1, G9 and G26 from Roodeplaat showed slightly higher WAC than their counterparts grown at Umbumbulu. High-water absorption of taro flour could be attributed to the presence of high amount of carbohydrates in these flours. Furthermore, Aboubakar et al. (2008) reported that the non-starch component of the flours such as the mucilage also contributes immensely to the water absorption of taro flours. In this study, amadumbe genotypes from Roodeplaat showed high WAC compared to their counterparts from Umbumbulu. This variation in WAC could be attributed to high level of mucilage in amadumbe genotypes from Roodeplaat. Flours with high WAC may be useful in products such as soups and gravies where superior viscosity is required (Kaushal et al. 2012).
Fig. 1.
Water and Oil absorption capacity of flours obtained from amadumbe genotypes grown in different locations. Error bars indicate standard deviation, G-genotype
Oil absorption capacity OAC of amadumbe flours was almost similar across all genotypes grown in both locations (Fig. 1). Environment or genotype did not seem to have any influence on the OAC, although slight differences were observed. Oil absorption capacity is useful in structure interaction in food especially in flavour retention, improvement of palatability and extension of shelf life particularly in bakery or meat products (Kaur et al. 2007).
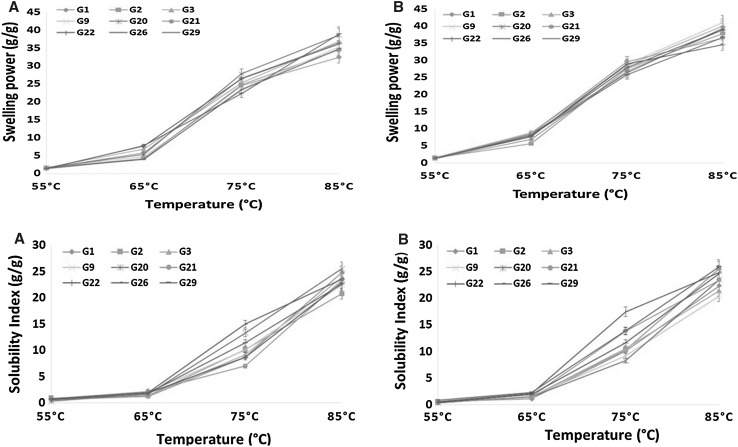
Swelling power Swelling power which is an indication of water absorption index of flours during heating was determined between 55 and 85 °C (Fig. 2) (Loos et al. 1981). At temperatures below 65 °C, the swelling power of amadumbe flours across all genotypes was relatively low. Between 65 and 85 °C degrees, rapid and continuous temperature dependent increase in swelling power was observed which can be attributed to gelatinisation of starch present in the flours (Hoover and Sosulski 1985). The genotype did not seem to have any significant effect on swelling power of amadumbe flours irrespective of growth location. Moorthy and Ramanujam (1986) suggested that the swelling power of granules reflected the extent of the associative forces within the granule. Genotypes from Roodeplaat seem to have higher swelling power than their counterparts grown in Umbumbulu. The higher SP for genotypes grown in Roodeplaat could be linked to inherent small starch granules (1–5 µm) (data not shown) contained within the flour. The smaller starch granules from Roodeplaat could have resulted from little rainfall received (514 mm) compared to those from Umbumbulu (828 mm). Soil water deficit have been reported to reduce the activities of enzymes involved in starch biosynthesis, resulting in inherent smaller starch granules, which consequently have an effect on swelling power (Zhong-Min et al. 2008). Furthermore, the lower swelling power of amadumbe could have been attributed to high protein content in flour isolated from amadumbe genotypes grown in Umbumbulu. According to Aprianita et al. (2009), proteins may cause the starch granules to be embedded within a stiff protein matrix which can subsequently limit access of the starch to water thereby restricting swelling capacity. In this study, a similar observation was also noted. Amadumbe flours with low protein and high carbohydrates seemed to have the higher swelling ability as previously reported (Kaur et al. 2013).
Fig. 2.
Swelling power and solubility index of flours isolated from amadumbe genotypes grown in different locations. a Amadumbe genotypes from Roodeplaat, *b Amadumbe genotypes from Umbumbulu, G genotype
Solubility index The solubility index was also determined within the temperature range of 55–85 °C. Temperature dependent increase in solubility was also observed across all amadumbe flours in both locations. The genotype seemed to influence the solubility index of flours within the temperature of 70–80 °C in both locations (Fig. 2). It was observed that the solubility pattern had a correlation with the swelling power. With the increase in swelling power, starch solubility also increased. Highest solubility and swelling power of flours were in the temperature range of 80–85 °C, which suggests maximum water penetration into the granules at elevated temperatures. The lower value of solubility of amadumbe flour might be due to the protein-amylose complex formation in amadumbe flour and isolated starch which agrees with findings reported in literature. Furthermore, Shimelis et al. (2006) also reported that starch and protein content of flour can interact due to the attraction of their opposite charges to form inclusion complexes during gelatinisation, which restricts swelling.
Pasting properties of flours The pasting temperatures (PT) of flours from amadumbe genotypes grown in both Roodeplaat and Umbumbulu ranged from approx 85–95 °C (Table 4). Flours isolated from amadumbe genotypes grown at Roodeplaat showed slightly higher PT (approx 91 °C) compared to the same genotypes from Umbumbulu (89 °C). Higher PT values of flours from Roodeplaat can also be attributed to high mucilage content of flours (Table 4). Mucilage has been reported to increase the pasting temperature of flour when it interacts with smaller amount of amylose released by the limited swelling of starch granules in flour (Jane et al. 1992; Liu et al. 2006). The highest pasting temperature of amadumbe flours in comparison to other flours indicate the presence of starch that is highly resistant to swelling and rupturing (Jane et al. 1992). Pasting temperature generally provides an indication of the minimum temperature required for sample cooking (Kaur et al. 2010).
Table 4.
Pasting profile of flours extracted from amadumbe genotypes grown in different locations
| Parameters | Pasting temperature (oC) | Peak Viscosity (RVU) | Breakdown (RVU) | Final Viscosity (RVU) | Setback (RVU) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Location | Roodeplaat | Umbumbulu | Roodeplaat | Umbumbulu | Roodeplaat | Umbumbulu | Roodeplaat | Umbumbulu | Roodeplaat | Umbumbulu |
| Genotypes | ||||||||||
| G1 | 92.4ab | 85.7b | 218ab | 188ab | 49.4bc | 70.9ab | 166c | 214a | 45.3c | 66.5a |
| G2 | 87.3c | 86.1b | 159c | 151b | 47.2bc | 32.6c | 155 cd | 174b | 46.1c | 56.5bc |
| G3 | 91.1bc | 85.9b | 195b | 197a | 57.3b | 53.3bc | 188b | 100 cd | 52.3b | 66.5a |
| G9 | 94.1a | 94.5a | 191b | 150b | 6.73f | 16.4e | 95.5e | 129c | 37.9d | 56.1bc |
| G20 | 91.7b | 86.9b | 161c | 133c | 41.5c | 22.8d | 176bc | 168b | 46.6c | 54.9bc |
| G21 | 90.9b | 86.8b | 139d | 148b | 44.4c | 78.5a | 129d | 215a | 33.8d | 64.8ab |
| G22 | 88.5c | 85.3b | 243a | 195a | 86.8a | 57.9b | 215a | 198ab | 61.3a | 61.9abc |
| G26 | 93.4ab | 94.5a | 83.4e | 54.5d | 17.7d | 10.3f | 124d | 76.1d | 45.5c | 22.9c |
| G29 | 92.8ab | 94.3a | 130d | 149b | 11.6e | 14.8e | 184b | 190ab | 46.3c | 52.8bc |
| Max | 94.1 | 94.5 | 242 | 197 | 86.8 | 78.5 | 215 | 215 | 61.3 | 66.5 |
| Min | 87.3 | 85.3 | 83.4 | 54.5 | 6.73 | 10.3 | 95.5 | 76.1 | 33.8 | 22.9 |
| C.V | 0.67 | 0.65 | 0.34 | 0.41 | 0.33 | 1.22 | 0.56 | 0.64 | 2.29 | 1.82 |
Mean ± SD Mean values with different letters in column are significantly different (p<0.05), Max maximum value, Min minimum value, C.V coefficient of value, G genotype, G with a number is the identification code of the genotype (Gn)
The peak viscosity (PV) of amadumbe flours varied significantly from approx. 83–243 RVU and 55–197 RVU for Roodeplaat and Umbumbulu, respectively (Table 4). On average, PV values of flour obtained from genotypes grown at Roodeplaat were higher (169 RVU) than those grown at Umbumbulu (152 RVU). Higher PV of flours from Roodeplaat can be attributed to lower temperatures (approx 19 °C) recorded during the growth season. Lower temperature has been reported to cause decrease in amylose content of the inherent starch molecules contained in the flour. Comparable results were found by Noda et al. (2001) who observed that PV of sweet potatoes clearly decreased with increase in soil temperature from (15–33 °C). Peak viscosity correlates with the quality of end-product and provides an indication of the viscous load likely to be encountered by a mixing cooker. Starch, mucilage and lipid contents of flours have been reported to influence the peak viscosity (Ragaee and Abdel-Aal 2006). In this study, genotypes, G1, G2, G9 and G22 grown at Roodeplaat and G3, G21 grown at Umbumbulu showed higher PV values, which can be attributed to high mucilage content of their flours (Table 4). Increased peak viscosity in the presence of a hydrocolloid has been reported in the literature (Huang et al. 2010; Liu et al. 2003). Flours with high PV are desirable especially in bread making since they produce a dough with great strength as previously reported in literature (Singh et al. 2016).
The breakdown viscosity (BD) of amadumbe flours was generally low across all genotypes grown in both locations. Genotypes, G9, G26 and G29 grown in both Roodeplaat and Umbumbulu generally showed lower BD compared to the rest of genotypes from both locations. Previous studies have reported that lower breakdown viscosity showed greater resistance which is normally expected of flours with lower peak viscosities and this was also evident in this study. Hence the higher the breakdown in viscosity, the lower the ability of the sample to withstand heating and shear stress during cooking (Adebowale et al. 2005).
The final Viscosity of amadumbe flours varied significantly among the genotypes. On average, amadumbe flours isolated from genotypes grown at Roodeplaat showed slightly higher final viscosity of 163 RVU in comparison to their counterparts grown at Umbumbulu (approx. 159 RVU). Final viscosity is used to define the quality of flour and indicates the stability of the cooked paste (Ikegwu et al. 2010). High final pasting viscosity results of amadumbe starch suggest that their starches can be potentially used as thickening agent in food applications.
Setback viscosity (SV) is associated with the tendency of starch to retrogradation (Owuamanam et al. 2010). The SV was generally low and varied significantly from approx. 23- 67 RVU among genotypes. Flours isolated from genotypes grown at Roodeplaat showed lower setback viscosity (approx. 6 RVU) compared to the same genotypes grown at Umbumbulu (approx. 56 RVU). When comparing with other tuber flours elsewhere, amadumbe flours in this study showed a lower setback indicating a lower tendency to retrograde (Kaushal et al. 2012). Lowest setback viscosity of amadumbe flours suggests a high resistance to retrogradation which is a positive indication for the formation of stable pastes. Lower SV is most likely caused by higher amylose content and shorter amylose chains present which cause intermolecular bonding associations (Hoover and Sosulski 1991).
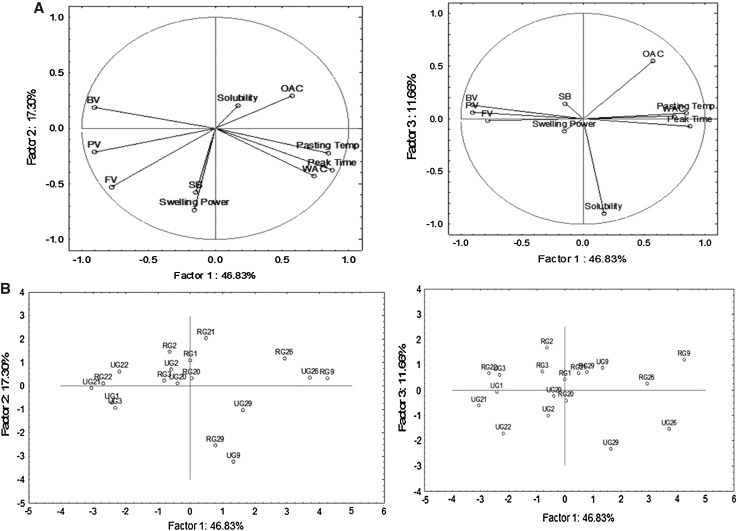
Principal component analysis Further, functional and pasting properties of flour from different growth locations were examined by principal component analysis (PCA). The first two components cumulatively explained 64% of the total variability of the data (Fig. 3). The first component accounted for approximately 47% of the variation and was able to separate amadumbe genotypes, G26 and G9 (from Roodeplaat) clustered on the right side of the plot from flour samples clustered on the left side (e.g. G22 from Roodeplaat and G21, G3, G1 from Umbumbulu). Flours samples on the left side of the plot were characterised by high peak viscosity, breakdown and final viscosity whilst those on the left side were characterised by high pasting temperature, high water and oil absorption capacities. The second component explained 17% of the total variation and separated G29 and G9 (from Umbumbulu) with high set back viscosity and swelling power from G2, G21, G20 and G3 (from Roodeplaat) which was characterised by high oil absorption capacities and low set back viscosities. The addition of the third component cumulatively explained 76% of the total variation of the data.
Fig. 3.
Principal component analysis for amadumbe flours. a showing vector loading for functional properties, Pasting Temp Pasting temperature, OAC-Oil absorption capacity, WAC Water absorption capacity, Swelling power, PTIME-Peak time, solubility, b showing the loading plot for genotypes from two locations, U Umbumbulu, R Roodeplaat
Conclusion
Amadumbe flour is an excellent source of carbohydrates, minerals and mucilage. Mineral composition varied significantly with genotypes and growth location. Functional properties such as swelling power, water absorption and pasting properties of flours were greatly influenced by carbohydrate and mucilage contents. Genotype and growth location significant affect the starch pasting properties of amadumbe flours. Findings from this study are important for future improvement programme and for food application of amadumbe flour. Amadumbe flour can be potentially used as food ingredient in food formulations for health-conscious individuals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank ARC for the financial support to carry out the study. Further appreciation goes to the University of Stellenbosch, Food Science department with the help of equipment used to analyse pasting properties.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2816-0) contains supplementary material, which is available to authorized users.
References
- Aboubakar Y, Njintang N, Scher J, Mbofung C. Physicochemical, thermal properties and microstructure of six varieties of taro (Colocasia esculenta L. Schott) flours and starches. J Food Eng. 2008;86(2):294–305. doi: 10.1016/j.jfoodeng.2007.10.006. [DOI] [Google Scholar]
- Adebowale A, Sanni L, Awonorin S. Effect of texture modifiers on the physicochemical and sensory properties of dried fufu. Food Sci Technol Inter. 2005;11(5):373–382. doi: 10.1177/1082013205058531. [DOI] [Google Scholar]
- AOAC I (2000) In Williams, S. (Ed.), Official methods of analysis. Washington DC: Association of Official Analytical Chemists
- Aprianita A. Assessment of underutilized starchy roots and tubers for their applications in the food industry. Victoria: Victoria University; 2010. [Google Scholar]
- Aprianita A, Purwandari U, Watson B, Vasiljevic T. Physicochemical properties of flours and starches from selected commercial tubers available in Australia. Int Food Res J. 2009;16:507–520. [Google Scholar]
- Falade KO, Okafor CA. Physical, functional, and pasting properties of flours from corms of two Cocoyam (Colocasia esculenta and Xanthosoma sagittifolium) cultivars. J Food Sci Technol. 2015;52(6):3440–3448. doi: 10.1007/s13197-014-1368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebresamuel N, Gebre-Mariam T. Comparative physico-chemical characterization of the mucilages of two cactus pears (Opuntia spp.) J Biomater Nanobiotechnol. 2012;3:79–86. doi: 10.4236/jbnb.2012.31010. [DOI] [Google Scholar]
- Guevara-Arauza JC, de Jesús Ornelas-Paz J, Pimentel-González DJ, Mendoza SR, Guerra RES, Maldonado LMTP. Prebiotic effect of mucilage and pectic-derived oligosaccharides from nopal (Opuntia ficus-indica) Food Sci Biotechnol. 2012;21(4):997–1003. doi: 10.1007/s10068-012-0130-1. [DOI] [Google Scholar]
- Hong G, Nip W. Functional properties of precooked taro flour in sorbets. Food Chem. 1990;36(4):261–270. doi: 10.1016/0308-8146(90)90065-C. [DOI] [Google Scholar]
- Hoover R, Sosulski FW. Studies on the functional, Characteristics and digestibility of starches from Phaseolus vulgaris biotypes. Starch Stärke. 1985;37:181–191. doi: 10.1002/star.19850370602. [DOI] [Google Scholar]
- Hoover R, Sosulski F. Composition, structure, functionality, and chemical modification of legume starches: a review. Can J Physiol Pharmacol. 1991;69(1):79–92. doi: 10.1139/y91-012. [DOI] [PubMed] [Google Scholar]
- Huang CC, Lai P, Chen IH, Liu YF, Wang CC. Effects of mucilage on the thermal and pasting properties of yam, taro, and sweet potato starches. LWT Food Sci Technol. 2010;43(6):849–855. doi: 10.1016/j.lwt.2009.11.009. [DOI] [Google Scholar]
- Ikegwu O, Okechukwu P, Ekumankana E. Physico-chemical and pasting characteristics of flour and starch from achi Brachystegia eurycoma seed. J Food Technol. 2010;8(2):58–66. doi: 10.3923/jftech.2010.58.66. [DOI] [Google Scholar]
- Jane J, Shen L, Chen J, Lim S, Kasemsuwan T, Nip W. Physical and chemical studies of taro starches and flours 1 2. Cereal Chem. 1992;69(5):528–535. [Google Scholar]
- Jiang G, Ramsden L. Characterisation and yield of the arabinogalactan–protein mucilage of taro corms. J Sci Food Agric. 1999;79(5):671–674. doi: 10.1002/(SICI)1097-0010(199904)79:5<671::AID-JSFA233>3.0.CO;2-H. [DOI] [Google Scholar]
- Kaur M, Singh N, Sandhu KS. Preparation and characterization of protein isolates from different lentil (Lens culinaris) cultivars. J Food SciTechnol. 2007;44(3):327–329. [Google Scholar]
- Kaur M, Sandhu KS, Lim ST. Microstructure, physicochemical properties and in vitro digestibility of starches from different Indian lentil (Lens culinaris) cultivars. Carbohydr Polym. 2010;79(2):349–355. doi: 10.1016/j.carbpol.2009.08.017. [DOI] [Google Scholar]
- Kaur M, Kaushal P, Sandhu KS. Studies on physicochemical and pasting properties of Taro (Colocasia esculenta L.) flour in comparison with a cereal, tuber and legume flour. J Food Sci Technol. 2013;50(1):94–100. doi: 10.1007/s13197-010-0227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal P, Kumar V, Sharma H. Comparative study of physicochemical, functional, antinutritional and pasting properties of taro (Colocasia esculenta), rice (Oryza sativa) flour, pigeon pea (Cajanus cajan) flour and their blends. LWT Food Sci Technol. 2012;48(1):59–68. doi: 10.1016/j.lwt.2012.02.028. [DOI] [Google Scholar]
- Liu H, Eskin NM, Cui SW. Interaction of wheat and rice starches with yellow mustard mucilage. Food Hydrocol. 2003;17(6):863–869. doi: 10.1016/S0268-005X(03)00107-3. [DOI] [Google Scholar]
- Liu H, Eskin NM, Cui SW. Effects of yellow mustard mucilage on functional and rheological properties of buckwheat and pea starches. Food Chem. 2006;95(1):83–93. doi: 10.1016/j.foodchem.2004.12.027. [DOI] [Google Scholar]
- Loos PJ, Hood L, Graham H. Isolation and characterization of starch from breadfruit (Artocarpus communis) Cereal Chem. 1981;58:83282–286. [Google Scholar]
- Mabhaudhi T, Modi Beletse Y. Response of taro (Colocasia esculenta L. Schott) landraces to varying water regimes under a rainshelter. Agric Water Manag. 2013;121:102–112. doi: 10.1016/j.agwat.2013.01.009. [DOI] [Google Scholar]
- Mergedus A, Kristl J, Ivancic A, Sober A, Sustar V, Krizan T, Lebot V. Variation of mineral composition in different parts of taro (Colocasia esculenta) corms. Food Chem. 2015;170:37–46. doi: 10.1016/j.foodchem.2014.08.025. [DOI] [PubMed] [Google Scholar]
- Moorthy S, Ramanujam T. Variation in properties of starch in cassava varieties in relation to age of the crop. Starch- Stärke. 1986;38(2):58–61. doi: 10.1002/star.19860380206. [DOI] [Google Scholar]
- Naidoo K, Amonsou E, Oyeyinka S. In vitro digestibility and some physicochemical properties of starch from wild and cultivated amadumbe corms. Carbohydr poly. 2015;125:9–15. doi: 10.1016/j.carbpol.2015.02.066. [DOI] [PubMed] [Google Scholar]
- Ndabikunze B, Talwana H, Issa-Zacharia A, Palapala V. Proximate and mineral composition of cocoyam (Colocasia esculenta L. and Xanthosoma sagittifolium L.) grown along the Lake Victoria Basin in Tanzania and Uganda. African. J Food Sci. 2011;5(4):248–254. [Google Scholar]
- Noda T, Kobayashi T, Suda I. Effect of soil temperature on starch properties of sweet potatoes. Carbohydr Polym. 2001;44(3):239–246. doi: 10.1016/S0144-8617(00)00227-7. [DOI] [Google Scholar]
- Owuamanam C, Ihediohanma N, Nwanekezi E. Sorption isotherm, particle size, chemical and physical properties of cocoyam corm flours. Researcher. 2010;2(8):11–19. [Google Scholar]
- Oyeyinka SA, Singh S, Amonsou EO. Physicochemical properties of starches extracted from bambara groundnut landraces. Starch- Stärke. 2016;69:3–4. [Google Scholar]
- Polycarp D, Afoakwa E, Budu A, Otoo E. Characterization of chemical composition and anti-nutritional factors in seven species within the Ghanaian yam (Dioscorea) germplasm. Int Food Res J. 2012;19(3):985–992. [Google Scholar]
- Ragaee S, Abdel-Aal ESM. Pasting properties of starch and protein in selected cereals and quality of their food products. Food Chem. 2006;95(1):9–18. doi: 10.1016/j.foodchem.2004.12.012. [DOI] [Google Scholar]
- Singh N, Kaur A, Katyal M, Bhinder S, Ahlawat AK, Singh AM. Diversity in quality traits amongst Indian wheat varieties II: Paste, dough and muffin making properties. Food Chem. 2016;197:316–324. doi: 10.1016/j.foodchem.2015.10.035. [DOI] [PubMed] [Google Scholar]
- Tattiyakul J, Asavasaksakul S, Pradipasena P. Chemical and physical properties of flour extracted from taro Colocasia esculenta (L.) Schott grown in different regions of Thailand. Sci Asia. 2006;32(3):279–284. doi: 10.2306/scienceasia1513-1874.2006.32.279. [DOI] [Google Scholar]
- Tester RF, Karkalas J. The effects of environmental conditions on the structural features and physicochemical properties of starches. Starch-Stärke. 2001;53(10):513–519. doi: 10.1002/1521-379X(200110)53:10<513::AID-STAR513>3.0.CO;2-5. [DOI] [Google Scholar]
- Thitisaksakul M, Jimenez RC, Arias MC, Beckles DM. Effects of environmental factors on cereal starch biosynthesis and composition. J Cereal Sci. 2012;56:67–80. doi: 10.1016/j.jcs.2012.04.002. [DOI] [Google Scholar]
- Zhong-Min D, Yan-Ping Y, Zhang M, Wen-Yang L, Su-Hui Y, Rui-Guo C, Zhen-Lin W. Distribution of starch granule size in grains of wheat grown under irrigated and rainfed conditions. Acta Agronomica Sinica. 2008;34(5):795–802. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.