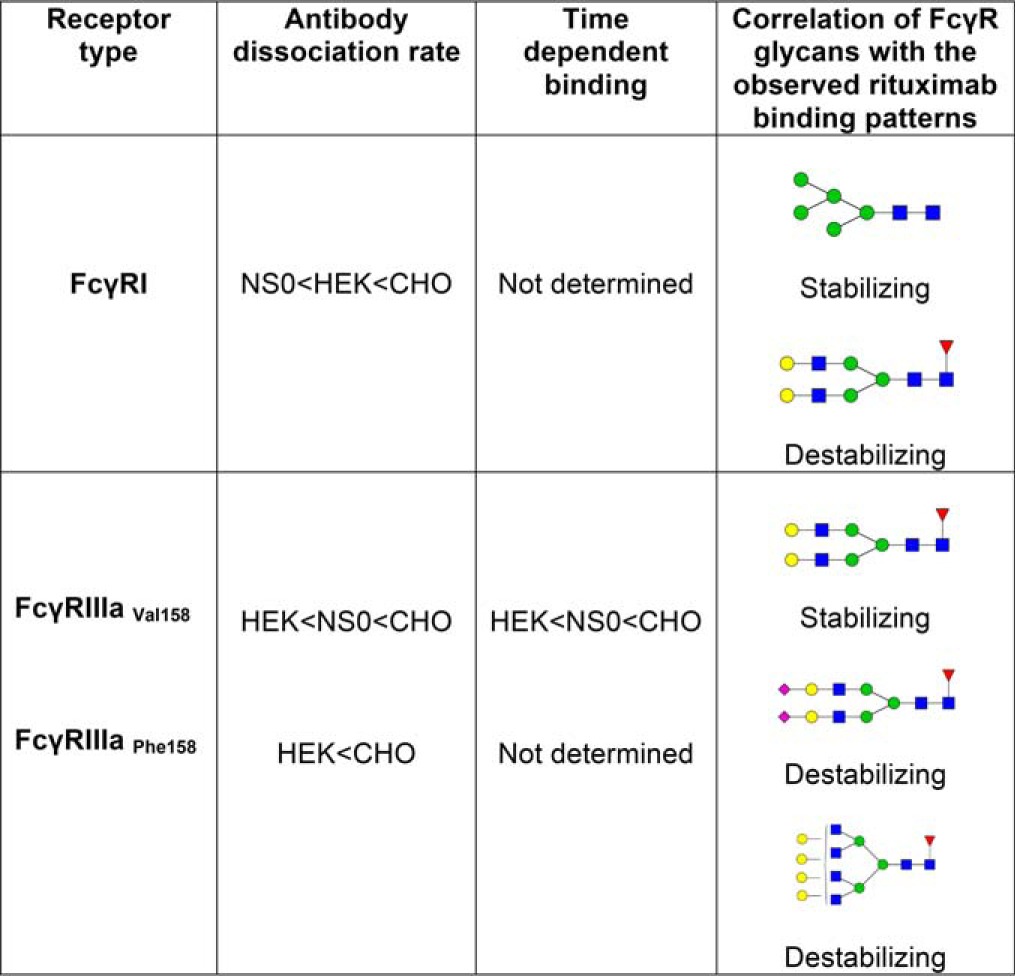
Table V. Correlation of FcγR-rituximab binding patterns with glycan profiles.
For FcγRI Man5 is found in the highest abundance on the NS0 receptor and lowest on the CHO receptor, matching the binding order with fastest dissociation from CHO-FcγRI. Man5 therefore appears to have a stabilizing effect on rituximab binding. The glycan FA2G2 is in the highest abundance on CHO FcγRI and together with other higher antennary glycan structures such as FA3G2 have a possible destabilizing effect on rituximab binding. For FcγRIIIa bi-antennary galactosylated glycans such as FA2G2 and FA2BG2 are the most abundant glycans and are also present in the highest amounts on HEK-FcγRIIIa, which has the slowest dissociation, which indicates that these glycans have a possible stabilizing effect on IgG1 binding to this receptor. Sialylation also appears to influence binding of rituximab to FcγRIIIa with CHO-FcγRIIIa having the highest amount of sialylation and the fastest dissociation. Higher amounts of sialylation and higher order sialylated structures are associated with less stable rituximab binding and increased rates of dissociation. Larger glycans such as FA4G4 also appear to have a destabilizing effect on IgG1 binding to FcγRIIIa.