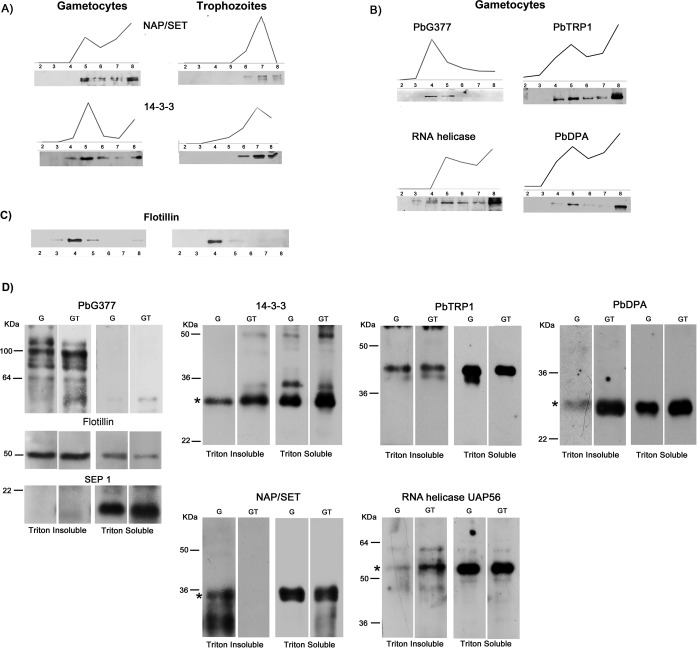
Fig. 5.
DRM partitioning of selected candidates. Floatation profile of selected proteins was determined either by label-free quantitative proteomics (PAP) or by Western blot (WB) analysis. Gradient fractions were probed with specific antibodies raised against: A, the nuclear protein NAP/SET and the nucleo-cytoplasmic protein 14-3-3, partially recruited into DRMs of both sexual and asexual stages; B, the gametocyte-specific protein PbG377, the putative RNA helicase UAP56, the conserved protein of unknown function PbTRP1 and the deoxyribose phosphate aldolase (PbDPA), recruited only in gametocyte DRMs. In all cases, WB profiles are superimposable to PAPs. in our experimental conditions, Flotillin resident into erythrocyte DRMs, floats to fraction 4 (C). To define membrane partioning of the selected proteins, specific antibodies were used in WB analysis of Triton-insoluble and -soluble extracts from nonactivated and 10 min-activated gametocytes (D). To validate the procedure, protein extracts were probed with antibodies against the DRM-resident proteins PbG377 and Flotillin and the integral membrane protein SEP1, not detected in DRM proteome. An asterisk indicates protein bands at the correct molecular mass. Multiple bands, were detected by PbG377-specific antibodies, because of post-translational cleavage.