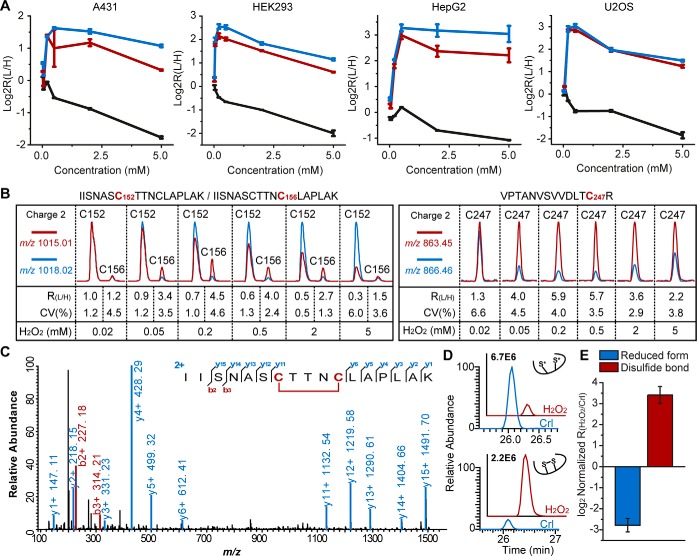
Fig. 4.
H2O2-dependent oxidation triggers dramatic structural changes of GAPDH. A, The measured light to heavy ratios of the peptides containing all three cysteines of GAPDH quantified by QTRP in four cell lines under H2O2 titration. C152, C156, and C247 were displayed in black, red, and blue color, respectively. B, Representative MS ion chromatograms from QTRP experiments for the peptides corresponding to all three cysteines of GAPDH in HEK293 cells. C, Representative MS/MS spectrum of a disulfide-bond (C152-C156) containing peptide from GAPDH in HEK293 cells. D, The PRM chromatographic traces of the disulfide-bond (C152-C156) containing peptide (lower) and its reduced form (upper; reduced cysteines were carbamidomethylated by iodoacetamide, depicted with asterisk) from endogenous GAPDH in HEK293 cells treated with or without H2O2. E, The relative abundance of GAPDH C152/C156 containing peptides in HEK293 cells treated with or without H2O2. To minimize the protein-level variation, the ratios of PRM peak areas of IISNAC152TTNC156LAPLAK in either disulfide bond or reduced form between H2O2-treated samples and controls were normalized with those obtained from an unmodified peptide (VIPELNGK) from GAPDH. For (A) and (E), data are presented as mean values ± S.D.; n = three biological replicates.