Abstract
Acute lymphoblastic leukemia (ALL) is a hematological malignancy characterized by abnormal proliferation and accumulation of lymphoblasts in the hematopoietic system. Stathmin 1 is a proliferation marker for normal lymphocytes, which has been described as highly expressed in ALL patients and functionally important for leukemia phenotype. In the present study, we expand our previous observations and aim to investigate Stathmin 1 expression and its impact on laboratory features and clinical outcomes in an independent cohort of ALL patients, and to verify the effects of paclitaxel treatment on Stathmin 1 phosphorylation and cell viability in ALL cell lines. In ALL patients, Stathmin 1 expression was significantly increased, associated with lower age onset and positively correlated with white blood cell counts, but did not impact on clinical outcomes. Functional assays revealed that paclitaxel induces Stathmin 1 phosphorylation at serine 16 (an inhibitory site), microtubule stability and apoptosis in Jurkat and Namalwa cell lines. Paclitaxel treatment did not modulate cell viability of normal peripheral blood leukocytes. In conclusion, our data confirm increased levels of Stathmin 1 in ALL patients and that therapeutic doses of paclitaxel inhibits Stathmin 1 function and promote microtubule stability and apoptosis in ALL cells.
Keywords: Medicine, Oncology, Cancer research, Pharmaceutical science, Cell biology
1. Introduction
Acute lymphoblastic leukemia (ALL) is a heterogeneous group of hematological malignancies characterized by abnormal proliferation and accumulation of lymphoblasts in the bone marrow, which impairs normal hematopoiesis [1]. Stathmin 1 is a phosphoprotein that participates in microtubule dynamics, cell cycle progression, proliferation and survival [2, 3]. Previously, our research group reported that Stathmin 1 is a proliferation marker for normal lymphocytes and that Stathmin 1 was overexpressed in a small cohort of ALL patients [4]. Similar findings were reported in lymphoma cell lines compared with non-transformed lymphoblastoid cells [5] and primary cells from high grade lymphoma and acute leukemia [6, 7]. Of note, Stathmin 1 silencing reduced cell proliferation and clonogenicity in an ALL cell line, supporting a functional role for Stathmin 1 in leukemia phenotype [4]. Stathmin 1 phosphorylation at serine 16 site reduces the affinity between Stathmin 1 and alpha/beta-tubulin heterodimers, inhibiting its microtubule destabilizing activity [2]. Recent studies have suggested that paclitaxel, a microtubule stabilizer drug, leads to Stathmin 1 inhibition by induction of serine 16 phosphorylation [8, 9].
We, herein, expand our previous observations and aim to investigate Stathmin 1 expression and its impact on clinical outcomes in an independent cohort of ALL patients, and to verify the effects of paclitaxel treatment on Stathmin 1 phosphorylation and cell viability in ALL cell lines.
2. Materials and methods
2.1. Primary samples
Bone marrow samples were collected from 22 healthy donors (median age 28 years [range 11–68]). Bone marrow or peripheral blood samples were collected from 45 ALL patients (median age 35 years [range 18–79]) at the time of diagnosis or relapse, followed up in the Clinic Hospital of our Institution (Table 1). Peripheral blood samples were collected from 4 healthy donors (median age 27 years [26–30]). The Ethics Committee of the Clinic Hospital of University of São Paulo at Ribeirão Preto Medical School approved this study and written informed consent was obtained from all subjects who participated in this study.
Table 1.
Patient's characteristcs.
| Parameters | Number |
|---|---|
| ALL patients | 45 |
| at diagnosis | 40 |
| at relapse | 5 |
| Sample | |
| Bone marrow (n), % blasts [median (range)] | 37 [86% (26–100)] |
| Peripheral blood (n), % blasts [median (range)] | 8 [68% (50–88%)] |
| Gender | |
| Male | 30 |
| Female | 15 |
| Age (years), median (range) | 35 (18–79) |
| Immunophenotyping | |
| T-ALL | 10 |
| B-ALL | 34 |
| Not available | 1 |
| BCR-ABL1 | |
| Positive | 10 |
| Negative | 35 |
Abbreviations: ALL, acute lymphoblastic leukemia; T-ALL, precursor T-acute lymphoblastic leukemia; B-ALL, precursor B-acute lymphoblastic leukemia; BCR-ABL1, breakpoint cluster region-abelson 1.
2.2. Quantitative PCR
Total RNA was obtained using the TRIzol reagent (Thermo Fisher Scientific, Fairlawn, NJ, USA). The cDNA was obtained from 1 μg of RNA using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). An aliquot of 120 ng of cDNA was used for gene expression analysis by quantitative polymerase chain reaction (qPCR) in the ABI 7500 Sequence Detection System (Thermo Fisher Scientific) using specific primers for Stathmin 1 (FW: AGCCCTCGGTCAAAAGAATC; RV: TTCAAGACCTCAGCTTCATGGG), HPRT1 (hypoxanthine phosphoribosyltransferase 1; forward: GAACGTCTTGCTCGAGATGTGA; reverse: TCCAGCAGGTCAGCAAAGAAT), ACTB (actin beta; forward: AGGCCAACCGCAAGAAG; reverse: ACAGCCTGGATAGCAACGTACA) and the SYBR™ Green Master Mix (Thermo Fisher Scientific). HPRT1 and ACTB were used as the reference gene. A negative ‘no template control’ was included for each primer pair. The dissociation protocol was performed at the end of each run to check for nonspecific amplification. Two replicas were run on the same plate for each sample. The relative gene expression was calculated using the equation 2−ΔΔCT [10].
2.3. Cell lines and functional assays
For functional assays, Jurkat (T-ALL) and Namalwa (B-ALL) cells were used (ATCC, Philadelphia, PA, USA). Cell lines were tested and authenticated by Short Tandem Repeat (STR) matching analysis and were mycoplasma-free. The IC50 was determined by methylthiazoletetrazolium (MTT) assay using cells treated with graded concentrations of paclitaxel (0, 0.625, 1.25, 2.5, 5, 10 and 20 nM; Sigma-Aldrich, St. Louis, MO, USA) for 72 h and estimated by nonlinear regression analysis. Apoptosis was determined by annexin V/PI staining according to manufacturer's instructions (BD Bioscience, San Jose, CA, USA).
2.4. Normal peripheral blood leukocyte culture
Normal peripheral blood leukocytes were obtained from healthy donors (n = 4) by Ficoll-Hypaque gradient separation (GE Healthcare, Roosendal, Netherlands), according to the manufacturer's instructions. Peripheral blood mononuclear cells were cultured on RPMI, supplemented with 30% fetal bovine serum, penicillin (100 U/mL), streptomycin (100 μg/mL), and IL3 (30 ng/mL), IL7 (100 ng/mL), FLT3 ligand (100 ng/mL), SCF (30 ng/mL), and at a confluence of 2 × 106 cells/mL. All human recombinant cytokines were from PeproTech (Rocky Hill, NJ, USA). Normal peripheral leukocytes were exposed to vehicle (DMSO), and paclitaxel at 5, 10 and 50 nM, and submitted to MTT assay and annexin V/PI staining.
2.5. Western blot
Western blot analyses were performed as previously described [4], using the SuperSignal™ West Dura Extended Duration Substrate System (Thermo Fisher Scientific) and Gel Doc XR+ system (Bio-Rad, Hercules, CA, USA). Antibodies against phospho(p)-Stathmin 1S16 (p-OP18S16; sc-12948-R), Stathmin 1 (OP-18, sc-55531), α-tubulin (sc-5286), and actin (sc-1616) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against acetyl-α-tubulinK40 (#5335S) and caspase 3 (#9665S) were purchased from Cell Signaling Technology (Danvers, MA, USA). Cropped gels retained important bands of one experiment, but whole gel images are available in Supplementary Fig. 1. Band intensities were determined using UN-SCAN-IT gel 6.1 software (Silk Scientific; Orem, UT, USA).
2.6. Statistical analysis
Statistical analyses were performed using GraphPad Instat 5 (GraphPad Software Inc., San Diego, CA, USA) or SAS Version 9.2 (SAS Inc, Cary, NC, USA). Mann–Whitney test or Student t test were used for measured factors. Fisher's exact test was used for categorical factors. Spearman test was used for ranking correlation tests. Kaplan-Meier method was used to estimate survival curves, which were compared by log-rank test. Cox regression analysis was used to estimate overall survival (OS), which was defined from time of sampling to date of death or last seen. A p value < 0.05 was considered as statistically significant.
3. Results and discussion
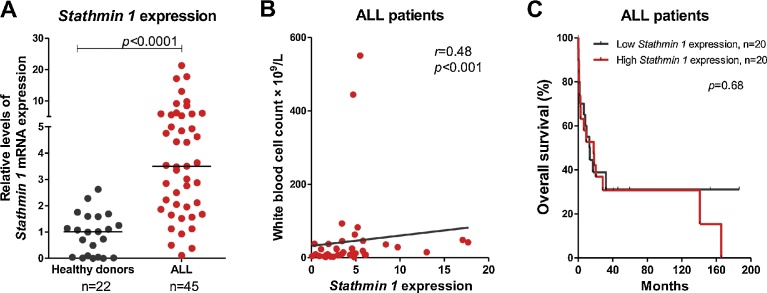
Stathmin 1 transcripts were significantly higher in primary cells from ALL patients compared with healthy donors (median = 3.50 [minimum = 0.11–maximum = 21.29] versus 1.01 [0.01–2.62], p < 0.0001; Fig. 1A). High Stathmin 1 expression was associated with lower age onset for ALL (Table 2) and Stathmin 1 levels positively correlated with white blood cell (WBC) count (r = 0.48; p = 0.001, Spearman test; Fig. 1B). However, Stathmin 1 expression did not impact on clinical outcomes of ALL patients (Fig. 1C and Table 3). Age significantly affected overall survival of our cohort. (Table 3). These findings validate our previous results on Stathmin 1 expression [4] in an independent cohort of ALL patients. Our data that Stathmin 1 expression does not impact on clinical outcomes needs to be confirmed in larger cohorts. Another research group showed that Stathmin 1 expression was correlated with WBC count and high percentages of cells in the S phase in a childhood ALL cohort [11]. In contrast, Stathmin 1 was found to be highly expressed in lymphoma and acute leukemia samples, but Stathmin 1 was not correlated with cells in the S phase [6]. Using proteomic approaches, another study observed Stathmin 1 overexpression in malignant B-cells from B-ALL patients compared to normal B-cells from healthy donors [12]. Taken together, these findings suggest that high Stathmin 1 expression is observed in ALL and plays a role in ALL biology, independently of its prognostic significance.
Fig. 1.
Stathmin 1 is highly expressed in primary acute lymphoblastic leukemia (ALL) samples. (A) qPCR analysis of Stathmin 1 mRNA levels in samples from ALL patients and healthy donors. The HPRT1 and ACTB genes were used as the reference gene and a healthy donor was used as a calibrator sample. Horizontal lines indicate medians. The numbers of subjects studied are indicated in the graph. The p-values are indicated; Mann-Whitney test. (C) Correlation analysis of Stathmin 1 expression and white blood cell count in ALL patients. The r and p-values are indicated; Spearman correlation test. (B) Overall survival of ALL patients categorized by the median of Stathmin 1 expression. Patients with ALL whose samples were collected at diagnosis (n = 40) were included in survival analysis. The p-values are indicated; Long-rank test.
Table 2.
Association between ALL patients’ clinical and laboratorial characteristics and Stathmin 1 expressiona.
| Parameters | All patients (n = 40) |
Stathmin 1 |
p-valueb | |
|---|---|---|---|---|
| Low expression (n = 20) | High expression (n = 20) | |||
| Age; median (range), y | 27 (18–79) | 37 (18–79) | 23 (18–78) | 0.01 |
| Female; n (%) | 12 (27.9) | 8 (40) | 4 (20) | 0.17 |
| Immunophenotype | 0.43 | |||
| B-ALL (%) | 32 (80) | 17 (85) | 15 (75) | |
| T-ALL (%) | 8 (20) | 3 (15) | 5 (25) | |
| BCR-ABL1 (%) | 8 (20) | 5 (25) | 3 (15) | 0.43 |
| Hemoglobin; median, (range); g/dL | 8.7 (5.2–15.8) | 8.1 (5.3–15.8) | 8.9 (5.2–12.8) | 0.79 |
| WBC; median (range) × 109/L | 13.7 (0.8–549.5) | 8.2 (1.1–92.1) | 20.65 (0.8–549.5) | 0.06 |
| Platelets; median (range) × 109/L | 36.5 (4–433) | 38 (7–433) | 32.5 (4–162) | 0.56 |
| LDH (range) U/L | 1789 (245–13090) | 1750 (245–6848) | 1789 (270–13090) | 0.32 |
Abbreviations: ALL, acute lymphoblastic leukemia; T-ALL, precursor T-acute lymphoblastic leukemia; B-ALL, precursor B-acute lymphoblastic leukemia; BCR-ABL1, breakpoint cluster region-abelson 1; WBC, white blood cell; LDH, lactic dehydrogenase.
Statistically significant p values are highlighted in bold.
Patients with ALL whose sample were collected at diagnosis (n = 40) were included in association analysis.
Mann–Whitney test and Fisher’s exact test were used for measured and categorical factors, respectively.
Table 3.
Univariate and multivariate analyses of overall survival for patients with ALLa.
| Factor | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| HRb | (95% C.I.) | p | HRb | (95% C.I.) | p | |
| Age | 1.04 | 1.02–1.07 | >0.001 | 1.05 | 1.02–1.08 | >0.001 |
| BCR-ABL1 (positive vs. negative) | 1.09 | 0.44–2.73 | 0.85 | 0.77 | 0.30–2.01 | 0.60 |
| WBC | 0.99 | 0.99–1.01 | 0.54 | 1.01 | 0.99–1.01 | 0.84 |
| Stathmin 1 expression | 1.01 | 0.92–1.11 | 0.82 | 1.05 | 0.96–1.16 | 0.25 |
Abbreviations: ALL, acute lymphoblastic leukemia; HR, hazard ratio; WBC, white blood cell; BCR-ABL1, breakpoint cluster region-abelson 1.
Statistically significant p values are highlighted in bold.
Patients with ALL whose sample were collected at diagnosis (n = 40) were included in survival analysis.
Hazard ratios >1 indicate that increasing values for continuous variable or the first factor for categorical variable has the poorer outcome.
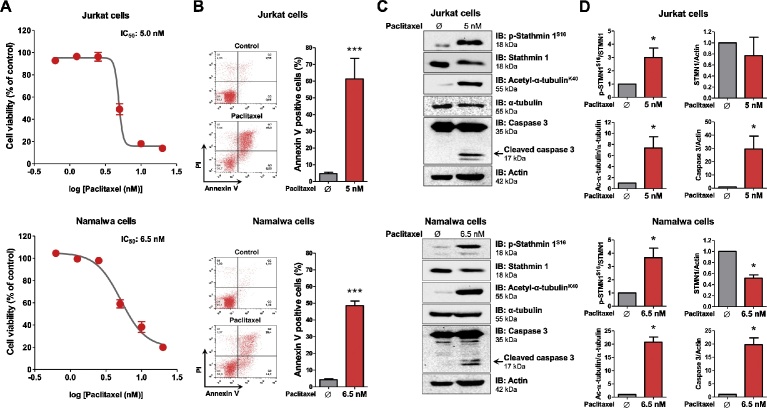
Cell viability and apoptosis assays reveal that Jurkat and Namalwa cells present high sensitivity to paclitaxel treatment (IC50 of 5 nM and 6.5 nM, respectively; Fig. 2A and B). Western blot analysis indicates that paclitaxel treatment strongly induces Stathmin 1 phosphorylation at serine 16 site (an inhibitory site), α-tubulin acetylation (a microtubule stability marker) and caspase 3 cleavage (an apoptosis marker) in ALL cell lines (Fig. 2C and D, Supplementary Fig. 1). In Namalwa cells, but not in Jurkat cells, paclitaxel significantly reduces Stathmin 1 expression (p < 0.05; Fig. 2C). Formerly, the induction of Stathmin 1 phosphorylation at serine 16 site upon paclitaxel treatment was observed in EC0156, an esophageal squamous cell carcinoma cell line [8]. Latterly, we observed a similar phenomenon in HEL, an acute myeloid leukemia cell line [9]. Paclitaxel is a microtubule-targeted chemotherapeutic drug used as the first line treatment for specific solid tumors [13]. According to studies performed in solid cancer patients, the plasma concentration of paclitaxel is higher than 50 nM [14, 15], which indicates a potential translational interest of this drug for ALL patients.
Fig. 2.
Paclitaxel reduces Stathmin 1 activity, microtubule instability and cell viability of ALL cell lines. (A) Dose-response cytotoxicity curves analyzed by methylthiazoletetrazolium (MTT) assay for Jurkat and Namalwa cells treated with graded doses of paclitaxel for 72 h. Values are expressed as the percentage of viable cells for each condition relative to untreated controls. Results are shown as the mean ± SD of four independent experiments. The IC50 was determined using nonlinear regression analysis. (B) Apoptosis was detected by flow cytometry in Jurkat or Namalwa cells treated with IC50 dose of paclitaxel (5 nM and 6.5 nM, respectively) for 72 h using annexin V/PI staining method. Representative dot plots are shown for each condition; the upper and lower right quadrants cumulatively contain the apoptotic population (annexin V+ cells). Bar graphs represent the mean ± SD of four independent experiments quantifying apoptotic cell death. ***p < 0.0001, Student t test. (C) Western blot analysis for phospho (p)-Stathmin 1S16, acetyl-α-tubulinK40 and caspase 3 (total and cleaved) in total cell extracts from Jurkat and Namalwa cells treated with the indicated concentrations of paclitaxel; membranes were reprobed with the antibody for the detection of the respective total protein or actin, and developed with the SuperSignal™ West Dura Extended Duration Substrate system using a Gel Doc XR+ imaging system. (D) Bar graphs represent the mean ± SD of three independent experiments quantifying band intensities of indicated proteins. *p < 0.05, Student t test. Note that paclitaxel treatment induces Stathmin 1 phosphorylation at serine 16 site (an inhibitory site), α-tubulin acetylation (a microtubule stability marker) and caspase 3 cleavage (an apoptosis marker) in Jurkat and Namalwa cells.
The mechanisms involved in aberrant Stathmin 1 expression in hematological malignancies remain unclear. It is known that oncogenes that induce a proliferative phenotype (e.g. BCR-ABL1) or inhibit cell differentiation (e.g. PML-RARα) induce Stathmin 1 expression in hematopoetic neoplasm models [16, 17]. Stathmin 1 knockout murine model is viable and has few mild hematological alterations [18]. On the other hand, Stathmin 1 inhibition results in a large reduction in cell proliferation and clonogenicity in human leukemia cell lines, suggesting selectivity for hematological malignant cells [4, 9, 19]. It is important to emphasize that drugs that disturb the microtubule dynamics, namely vincristine, have already been used in the treatment of ALL [20]. Thus, the understanding of the molecular bases of microtubule dynamics and the identification of key proteins involved in this cellular process may provide new therapy opportunities for leukemia.
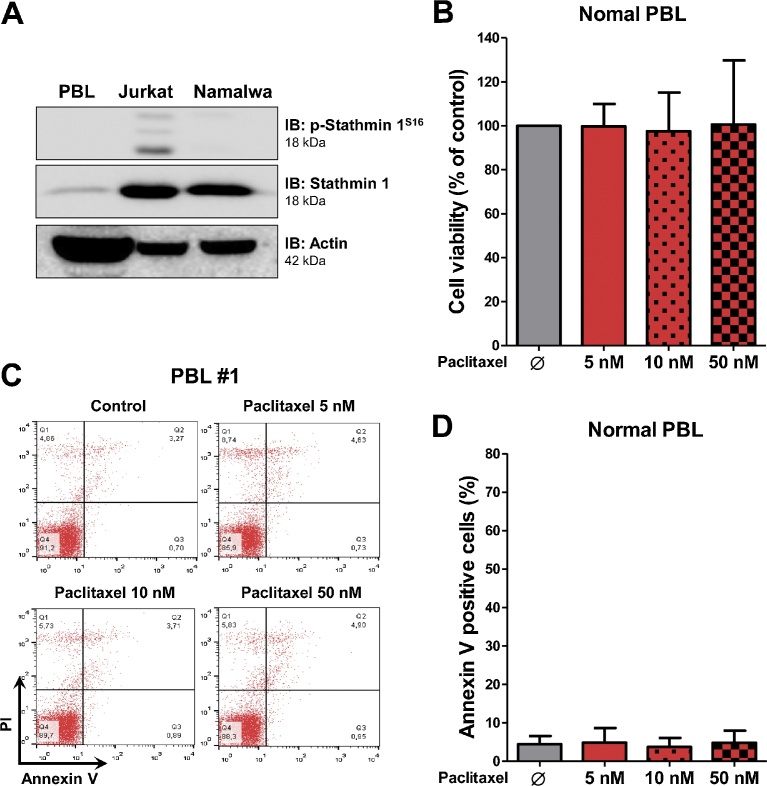
Stathmin 1 expression and phosphorylation were reduced in peripheral blood mononuclear cells from healthy donors compared to Jurkat and Namalwa cells (Fig. 3A, Supplementary Fig. 1). Our research group already reported that Stathmin 1 has very low expression, at mRNA and protein levels, in normal blood cells, including lymphocytes, monocytes and granulocytes [4]. Similar findings were also reported for mature erythrocytes [21] and platelets [22]. Of note, paclitaxel treatment did not modulate cell viability in peripheral blood leukocytes from healthy donors (Fig. 3B and D), even at doses compatible with high serum levels achieved in pharmacodynamic experiments [14, 15].
Fig. 3.
Paclitaxel do not modulate cell viability of normal peripheral blood leukocytes. (A) Western blot analysis for phospho (p)-Stathmin 1S16 and Stathmin 1 in total cell extracts from normal peripheral blood leukocytes, Jurkat and Namalwa cells; membranes were reprobed with the antibody for the detection of the respective total protein or actin, and developed with the SuperSignal™ West Dura Extended Duration Substrate system using a Gel Doc XR+ imaging system. (B) Bar graphs represent mean ± SD of cell viability analyzed by methylthiazoletetrazolium (MTT) assay for normal peripheral blood leukocytes (n = 4) treated with vehicle, and paclitaxel at 5, 10 and 50 nM for 72 h. Values are expressed as the percentage of viable cells for each condition relative to untreated controls. (C) Apoptosis was detected by flow cytometry in normal peripheral blood leukocytes treated with vehicle, and paclitaxel at 5, 10 and 50 nM for 72 h using annexin V/PI staining method. A representative dot plot is shown for each condition; the upper and lower right quadrants cumulatively contain the apoptotic population (annexin V+ cells). (D) Bar graphs represent the mean ± SD of independent experiments from normal peripheral blood leukocytes (n = 4) treated with vehicle, and paclitaxel at 5, 10 and 50 nM for 72 h.
In conclusion, our data confirm increased levels of Stathmin 1 in an independent cohort of ALL patients, indicating that Stathmin 1 may participate in leukemia phenotype. Stathmin 1 expression did not impact ALL outcomes. Paclitaxel promotes Stathmin 1 phosphorylation at serine 16 site, microtubule stability and apoptosis in ALL cell lines. Further studies are necessary to identify the molecular mechanisms involved in the paclitaxel-induced phosphorylation of Stathmin 1.
Declarations
Author contribution statement
João Agostinho Machado-Neto: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Ana Paula Nunes Rodrigues Alves, Jaqueline Cristina Fernandes, Juan Luiz Coelho-Silva, Renata Scopim-Ribeiro, Bruna Alves Fenerich, Fernanda Borges da Silva, Priscila Santos Scheucher: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Belinda Pinto Simões, Eduardo Magalhães Rego: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Fabiola Traina: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Competing interest statement
The authors declare no conflict of interest.
Funding statement
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank Andy Cumming for English review.
Contributor Information
João Agostinho Machado-Neto, Email: jamachadoneto@gmail.com.
Fabiola Traina, Email: ftraina@fmrp.usp.br.
Appendix A. Supplementary data
Western blot analysis for phopho (p)-Stathmin 1S16, acetyl-α-tubulinK40 and caspase 3 (total and cleaved) in total cell extracts from Jurkat and Namalwa cells treated with the indicated concentrations of paclitaxel or for p-Stathmin 1S16 and Stathmin 1 in total cell extracts from normal peripheral blood leukocytes, Jurkat and Namalwa cells; membranes were reprobed with the antibody for the detection of the respective total protein or actin, and developed with the SuperSignal™ West Dura Extended Duration Substrate system using a Gel Doc XR+ imaging system.
References
- 1.Paul S., Kantarjian H., Jabbour E.J. Adult acute lymphoblastic leukemia. Mayo Clin. Proc. 2016;91:1645–1666. doi: 10.1016/j.mayocp.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Belletti B., Baldassarre G. Stathmin: a protein with many tasks. New biomarker and potential target in cancer. Expert Opin. Ther. Targets. 2011;15:1249–1266. doi: 10.1517/14728222.2011.620951. [DOI] [PubMed] [Google Scholar]
- 3.Biaoxue R., Xiguang C., Hua L., Shuanying Y. Stathmin-dependent molecular targeting therapy for malignant tumor: the latest 5 years' discoveries and developments. J. Transl. Med. 2016;14:279. doi: 10.1186/s12967-016-1000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machado-Neto J.A., de Melo Campos P., Favaro P., Lazarini M., Lorand-Metze I., Costa F.F., Olalla Saad S.T., Traina F. Stathmin 1 is involved in the highly proliferative phenotype of high-risk myelodysplastic syndromes and acute leukemia cells. Leuk. Res. 2014;38:251–257. doi: 10.1016/j.leukres.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Brattsand G., Roos G., Marklund U., Ueda H., Landberg G., Nanberg E., Sideras P., Gullberg M. Quantitative analysis of the expression and regulation of an activation-regulated phosphoprotein (oncoprotein 18) in normal and neoplastic cells. Leukemia. 1993;7:569–579. [PubMed] [Google Scholar]
- 6.Roos G., Brattsand G., Landberg G., Marklund U., Gullberg M. Expression of oncoprotein 18 in human leukemias and lymphomas. Leukemia. 1993;7:1538–1546. [PubMed] [Google Scholar]
- 7.Nylander K., Marklund U., Brattsand G., Gullberg M., Roos G. Immunohistochemical detection of oncoprotein 18 (Op18) in malignant lymphomas. Histochem. J. 1995;27:155–160. doi: 10.1007/BF00243911. [DOI] [PubMed] [Google Scholar]
- 8.Liu F., Sun Y.L., Xu Y., Wang L.S., Zhao X.H. Expression and phosphorylation of stathmin correlate with cell migration in esophageal squamous cell carcinoma. Oncol. Rep. 2013;29:419–424. doi: 10.3892/or.2012.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machado-Neto J.A., de Melo Campos P., Favaro P., Lazarini M., da Silva Santos Duarte A., Lorand-Metze I., Costa F.F., Saad S.T., Traina F. Stathmin 1 inhibition amplifies ruxolitinib-induced apoptosis in JAK2V617F cells. Oncotarget. 2015;6:29573–29584. doi: 10.18632/oncotarget.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 11.Melhem R., Hailat N., Kuick R., Hanash S.M. Quantitative analysis of Op18 phosphorylation in childhood acute leukemia. Leukemia. 1997;11:1690–1695. doi: 10.1038/sj.leu.2400792. [DOI] [PubMed] [Google Scholar]
- 12.Saha S., Banerjeea S., Banerjeec D., Chandrac S., Chakrabartia A. 2DGE and DIGE based proteomic study of malignant B-cells in B-cell acute lymphoblastic leukemia. EuPA Open Proteom. 2014;3:13–26. [Google Scholar]
- 13.Jordan M.A., Wilson L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 14.Zasadil L.M., Andersen K.A., Yeum D., Rocque G.B., Wilke L.G., Tevaarwerk A.J., Raines R.T., Burkard M.E., Weaver B.A. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci. Transl. Med. 2014;6:229–243. doi: 10.1126/scitranslmed.3007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gianni L., Kearns C.M., Giani A., Capri G., Vigano L., Lacatelli A., Bonadonna G., Egorin M.J. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J. Clin. Oncol. 1995;13:180–190. doi: 10.1200/JCO.1995.13.1.180. [DOI] [PubMed] [Google Scholar]
- 16.Unwin R.D., Sternberg D.W., Lu Y., Pierce A., Gilliland D.G., Whetton A.D. Global effects of BCR/ABL and TEL/PDGFRbeta expression on the proteome and phosphoproteome: identification of the Rho pathway as a target of BCR/ABL. J. Biol. Chem. 2005;280:6316–6326. doi: 10.1074/jbc.M410598200. [DOI] [PubMed] [Google Scholar]
- 17.Zada A.A., Geletu M.H., Pulikkan J.A., Muller-Tidow C., Reddy V.A., Christopeit M., Hiddemann W.D., Behre H.M., Tenen D.G., Behre G. Proteomic analysis of acute promyelocytic leukemia: PML-RARalpha leads to decreased phosphorylation of OP18 at serine 63. Proteomics. 2006;6:5705–5719. doi: 10.1002/pmic.200600307. [DOI] [PubMed] [Google Scholar]
- 18.Ramlogan-Steel C.A., Steel J.C., Fathallah H., Iancu-Rubin C., Soleimani M., Dong Z., Atweh G.F. The role of stathmin, a regulator of mitosis, in hematopoiesis. Blood (ASH Ann. Meet. Abstr.) 2012;120 Abstract #3453. [Google Scholar]
- 19.Iancu C., Mistry S.J., Arkin S., Wallenstein S., Atweh G.F. Effects of stathmin inhibition on the mitotic spindle. J. Cell Sci. 2001;114:909–916. doi: 10.1242/jcs.114.5.909. [DOI] [PubMed] [Google Scholar]
- 20.Terwilliger T., Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7:e577. doi: 10.1038/bcj.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabilloud T., Berthier R., Valette C., Garin J., Lawrence J.J. Induction of stathmin expression during erythropoietic differentiation. Cell Growth Differ. 1995;6:1307–1314. [PubMed] [Google Scholar]
- 22.Rubin C.I., French D.L., Atweh G.F. Stathmin expression and megakaryocyte differentiation: a potential role in polyploidy. Exp. Hematol. 2003;31:389–397. doi: 10.1016/s0301-472x(03)00043-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Western blot analysis for phopho (p)-Stathmin 1S16, acetyl-α-tubulinK40 and caspase 3 (total and cleaved) in total cell extracts from Jurkat and Namalwa cells treated with the indicated concentrations of paclitaxel or for p-Stathmin 1S16 and Stathmin 1 in total cell extracts from normal peripheral blood leukocytes, Jurkat and Namalwa cells; membranes were reprobed with the antibody for the detection of the respective total protein or actin, and developed with the SuperSignal™ West Dura Extended Duration Substrate system using a Gel Doc XR+ imaging system.