Fig. 3.
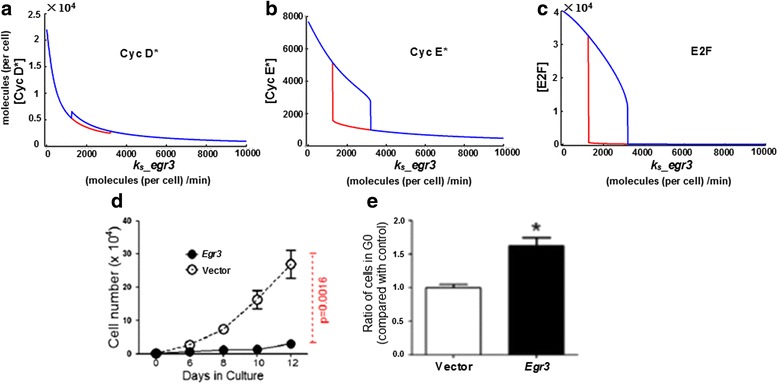
Simulations of system dynamics with respect to Egr3 expression. a-c Stable equilibriums (steady states) of Cyc D*, Cyc E* and E2F with respect to the expression level of Egr3 are shown in (a), (b) and (c) respectively. Similar to Fig. 2a–c, data are steady state concentrations under various genetic synthesis rates (k s_egr3, i.e. expression level) of Egr3. Stable equilibriums of all the three cell cycle checkpoint-determining molecules have shifted downward as k s_egr3 increases, especially for Cyc E* b and E2F c as their steady states decline sharply in the manner of bistability (i.e. low-level stable equilibriums occur due to dynamical bifurcation; red lines). The steady states of Cyc D* exhibit very slight upward fluctuations at certain Egr3 expression levels because the decreases in E2F at those k s_egr3 values result in less amounts of p18 and p19 (inhibitors of Cyc D*), thus causing the transient elevation. However, the Cyc D* level rapidly declines as k s_egr3 further increases. Noteworthy, when k s_egr3 exceeds certain values, the three molecules are all suppressed to very low levels, indicating that high Egr3 expression potently suppresses cell cycle. d Proliferation of HSCs over-expressing (i.e. transducted with) Egr3; similar to Fig. 2d, “Vector” stands for control data. As shown, the proliferation capability of HSCs highly expressing Egr3 is significantly lowered (p-value <0.005). e Ratio of HSCs staying in G0 phase before (control) and after Egr3 over-expression. Data show that the ratio of G0 HSCs is significently heightened when Egr3 is highly expressed (p-value <0.05), indicating that high Egr3 expression arrests HSCs in the G0 phase, reducing the number of HSCs entering the later phase(s) of cell cycle
