Fig. 8.
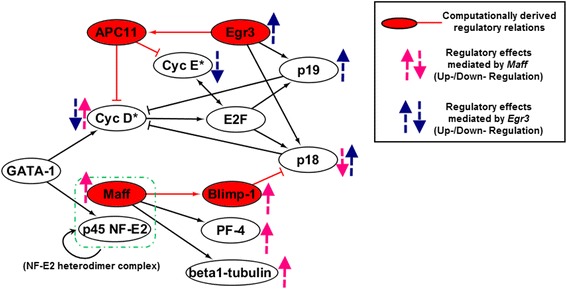
Molecular regulations of cell cycle and hematopoiesis. GATA-1 (Gata-1) activates the cell cycle components as well as p45 NF-E2, the heterodimer partner of Maff. The Maff:p45 NF-E2 functional complex is self-regulated and it activates the transcriptions of various downstream regulators, e.g. Blimp-1 (Blimp1), β1-tubulin (Tubb1), and PF-4 (Pf4). Altogether with our prediction that “Maff − ⊣ p18”, it can be concluded that high expression of Maff eliminates the feedback inhibtion of p18 to Cyclin D:Cdk4/6, as well as up-regulating the downstream TFs. Hence, cell cycle will be accelerated and proliferation/differentiation of hematopoietic cells are enhanced. This is why we hypothesize that high expression of Maff adapts cells to a rapidly proliferative status, i.e. cancerization. On the other side, Egr3 suppresses the checkpoint controllers of G0 → G1/G1 → S and activates CKIs, thus its high expression greatly inhibits cell cycle. Thus it is fairly assumed that it may be a “harsh control” via which HSCs forcibly shut down functionalities when their behaviors exceed the limits of cellularity controlling principles, i.e. self-protection
