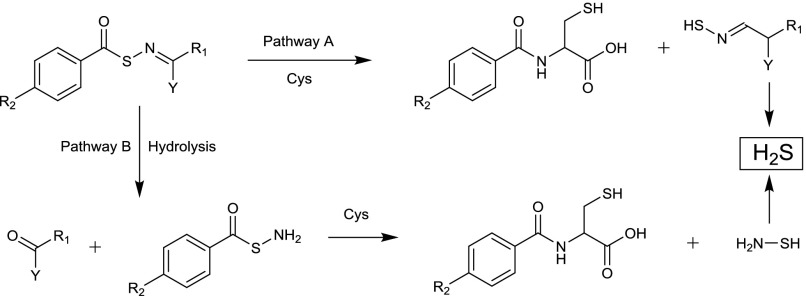
Fig. 14.
Mechanisms of H2S release from S-aroylthiooximes (SATOs). Two pathways could lead to H2S generation from SATOs. The first involving addition of the cysteine thiol to the SATO acyl group followed by rapid S→N-acyl transfer (Pathway A). The arylidenethiooxime would form, which could decompose to generate a ketone or aldehyde along with H2S and NH3. The second pathway involves a hydrolysis step to generate the S-aroylthiohydroxylamine and the ketone or aldehyde used to make the SATO (Pathway B). The fast reaction between SATOs and cysteine to yield H2S (t1/2 approximately 8−82 minutes) compared with the hydrolysis rate (t1/2 ranging from 45–250 hours) rules out pathway B as the mechanism of H2S release mechanism for most SATOs under the conditions tested in Foster et al., 2014.