Abstract
Knowledge about interactions between reward and negative processing is rudimentary. Here, we employed functional MRI to probe how potential reward signaled by advance cues alters aversive distractor processing during perception. Behaviorally, the influence of aversive stimuli on task performance was reduced during the reward compared to no-reward condition. In the brain, at the task phase, paralleling the observed behavioral pattern, we observed significant interactions in the anterior insula and dorsal anterior cingulate cortex, such that responses during the negative (vs neutral) condition were reduced during the reward compared to no-reward condition. Notably, negative distractor processing in the amygdala appeared to be independent of the reward manipulation. During the initial cue phase, we observed increased reward-related responses in the ventral striatum/accumbens, which were correlated with behavioral interference scores at the subsequent task phase, revealing that participants with increased reward-related responses exhibited a greater behavioral benefit of reward in reducing the adverse effect of negative images. Furthermore, during processing of reward (vs no-reward) cues, the ventral striatum exhibited stronger functional connectivity with fronto-parietal regions important for attentional control. Together, our findings contribute to the understanding of how potential reward influences attentional control and reduces negative distractor processing in the human brain.
Keywords: reward, emotion, perception, ventral striatum, amygdala
Introduction
In many real life situations, appetitive and aversive information jointly impact brain and behavior. Understanding potential interactions between them is important given their relevance to disorders such as depression and anxiety, where disturbances in reward and negative processing are well documented (Dillon et al., 2014; Pizzagalli, 2014). Brain imaging studies that have investigated interactions between reward and aversive processing have mainly focused on decision-making mechanisms (Talmi et al., 2009; Park et al., 2011); a few studies have also probed the influence of stress on different aspects of reward processing (Ossewaarde et al., 2011; Porcelli et al., 2012; Kumar et al., 2014). Notably, most investigations have focused on understanding the influence of aversive processing on reward mechanisms, but not vice versa (Wittmann et al., 2008). Thus, it is important to probe the influence of reward on negative processing, in particular during perception and attention tasks.
Recent work suggests that potential reward-driven motivation enhances executive control mechanisms that help with prioritizing goal-relevant information, leading to improved performance across perceptual and cognitive tasks (Pessoa, 2013; Botvinick and Braver, 2015). For instance, using a proactive manipulation, where the potential for reward was signaled by advance cues, we reported that enhanced selective attention during the reward condition reduced the influence of neutral distractors resulting in better performance during a Stroop-like interference task (Padmala and Pessoa, 2011). In a similar fashion, potential reward has been shown to reduce switching costs in task-switching paradigms (Savine et al., 2010), decrease stop-signal reaction times during response inhibition tasks (Boehler et al., 2012), and increase post-conflict control in flanker tasks (Braem et al., 2012). These and other recent findings in the literature highlight the role of reward motivation in fine tuning executive function in a manner that ultimately leads to selective effects in behavior.
However, little is known about how potential reward-driven attentional control mechanisms impact the processing of task-irrelevant negative information. Two event-related potential (ERP) studies investigated this question, but yielded mixed results. One study claimed independence between reward expectancy and negative information and suggested that emotion-related processing was privileged and immune to the reward manipulation (Kaltwasser et al., 2013). In contrast, a second study reported that reward expectation reduced the processing of task-irrelevant negative information (Wei et al., 2016). Overall, our knowledge about the mechanisms underlying the influence of reward expectancy on aversive distractor processing is incomplete.
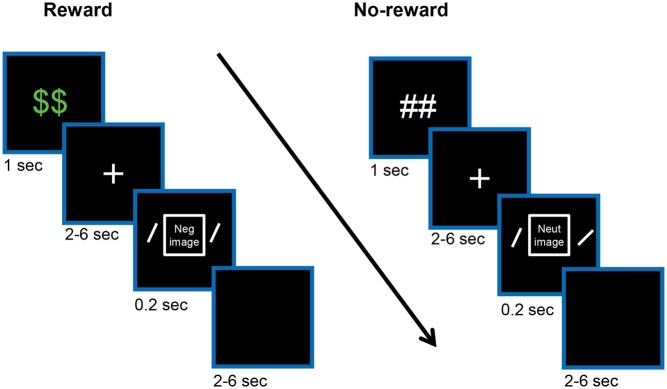
In the present study, we investigated the influence of performance-based rewards on aversive distractor processing with functional MRI. As in our recent behavioral study (Padmala and Pessoa, 2014), we used a proactive manipulation of reward, where each trial started with an advance cue that signaled the potential for reward. During the task phase, participants discriminated the orientation of peripheral bars while ignoring a centrally presented aversive or neutral picture (Figure 1). In our previous behavioral study, we detected a interaction between potential reward and negative items, where the interference of negative distractors was reduced with reward expectancy (Padmala and Pessoa, 2014). We thus anticipated that, in the present study, reward expectancy would reduce the impact of negative distractors at the behavioral level.
Fig. 1.
Experimental design. On each trial, an initial cue indicated potential for reward followed by a variable delay period during which a white fixation cross was shown. Then a negative or neutral task-irrelevant picture (not shown here) was presented centrally and two bars were presented peripherally (not drawn to scale). The participant’s task was to indicate whether the bars are of same or different orientation while ignoring the central picture. Finally, each trial ended with a variable inter-trial interval. During the reward condition (left side), participants were rewarded if performance was both fast and accurate.
Analogous to behavior, we also anticipated interactions between potential reward-driven goal-directed attentional control and negative distractor processing in the brain during the task phase. We were particularly interested in evaluating responses in the amygdala to test for potential mechanisms of interaction. An interaction pattern revealing reduced differential responses to negative vs neutral pictures during the reward condition would indicate that potential reward helped in filtering out distractors (and would be consistent with the anticipated behavioral effect). In contrast, the lack of an interaction pattern in the amygdala in the presence of the anticipated behavior (decreased negative distractor interference with reward), would provide evidence that negative stimuli were not filtered out, but that enhanced attentional control still was able to counteract their adverse influence.
Furthermore, we hypothesized that the processing of a reward cue would lead to increased responses in the ventral striatum, as well as fronto-parietal regions important for attention (Botvinick and Braver, 2015). We evaluated two (not mutually exclusive) forms of brain-behavior links. If ventral striatum activity reflected a ‘reward signal’, cue-related responses would be associated with a general energizing effect of reward expectancy on behavior (reward main-effect scores across participants); in contrast, if ventral striatum activity reflected more specific processes allowing improved behavior during challenging situations (see Sarter et al., 2006; Salamone et al., 2009), cue-related responses would be associated with a selective effect of potential reward on behavior (as indexed via reward by emotion interaction scores).
Materials and methods
For full information on Methods, please see Supplementary Information.
Participants
Sixty-one participants (31 males; mean age: 21.7 years, range: 18–33 years) provided consent to participate in the study, which was approved by the Institutional Review Board of the University of Maryland, College Park. After exclusion, data from 57 participants were analyzed.
Stimuli and behavioral paradigm
Each trial (Figure 1) started with the presentation (1 s) of a cue stimulus indicating the Reward condition (‘##’: no-reward; ‘$$’: reward). The cue was followed by a 2–6 s variable delay period during which a white fixation cross was shown at the center of the screen. Then, a centrally positioned image (neutral or negative; 4° × 4°) was shown (0.2 s) together with two oriented bars positioned peripherally (3.5° degrees to the left and right). The participant’s task was to indicate whether the two bars were of same or different orientation and were given 1500 ms to respond via button press. The central images were task irrelevant. Forty-eight neutral and 48 negative images were employed from the International Affective Picture System (Lang et al., 2008.) and a database of mutilation images developed by the Laboratory of Neurophysiology of Behavior at the Federal Fluminense University, Brazil (Mocaiber et al., 2011). The trial ended with a 2–6 s variable blank screen. Participants performed a total of six runs of 32 trials each presented in random order, totaling 48 trials per condition for the entire experiment. Participants were informed that they could earn 25 cents per trial during the reward condition if they were both fast and accurate.
Voxelwise analysis
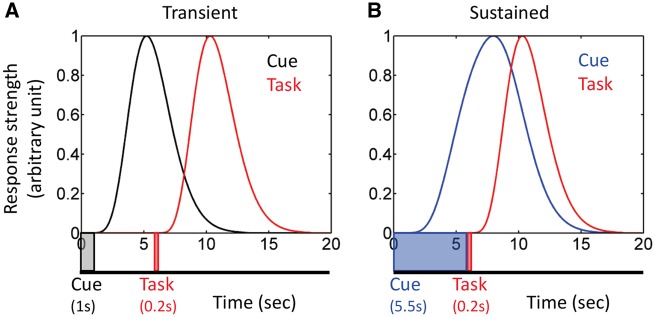
Each participant’s preprocessed functional MRI data were analyzed using multiple linear regression with AFNI using the 3dDeconvolve program. Our design included, on each trial, a variable delay between the cue and task phases and a variable inter-trial interval. When cue-phase responses are transient and independent of signals during the cue-to-task delay, this kind of design enables estimation of separate cue and task responses (Serences, 2004). However, in the present experiment, it is conceivable that cue responses would be sustained during the delay period, in particular in regions involved in preparatory attention (Engelmann et al., 2009). In this scenario, because cue-phase responses might be present until the onset of the task phase (Figure 2), estimation of unique contributions of the two phases is problematic because of the considerable response overlap (Ruge et al., 2009); note that the overlap would be present for short or long delay periods, that is, it is independent of delay duration. To address this issue, we estimated cue and task responses using a two-step procedure, as follows.
Fig. 2.
Simulated functional MRI responses and potential cue-task overlap. (A) To simulate transient cue responses, the cue was assumed to be presented for 1 s (as in the experiment), followed by a 4.5-s delay period (which varied between 2 and 6 s in the experiment), and a 0.2-s task phase (indicated by the timeline). The overlap between cue and task responses is small. (B) To simulate sustained cue responses, the cue was assumed to the presented for 5.5 s and followed immediately by a 0.2-s task phase (indicated by the timeline). The overlap between cue and task responses is quite substantial. Simulated responses were generated by employing a canonical gamma variate hemodynamic response function (Cohen, 1997).
First, we estimated cue-phase responses after controlling for task-related activity. To do so, we ran multiple regression on the preprocessed functional data including only task phase regressors in the model: no-reward and reward events at the task phase separately for the neutral and negative conditions, and a separate regressor of no interest that modeled error trials (pooled over all four conditions) during the task phase. Additionally, to model baseline and drifts of the MR signal, constant, linear, and quadratic terms were included for each run separately as covariates of no interest; six estimated motion parameters and their first derivatives were also included as regressors in the model. Subsequently, the residual time series from this initial multiple regression model was used as input for a subsequent multiple regression analysis which included only cue phase regressors: reward and no-reward events at the cue phase, and error trials (pooled over all four conditions) at the cue phase. In this manner, estimates of cue phase responses from the second model were uncontaminated from task-phase responses. Likewise, task phase responses were estimated after controlling for cue-related activity.
As a control analysis to confirm that the results were not unduly related to the two-step procedure, we ran a separate multiple regression analysis which included both cue and task phase regressors in the same model: no-reward and reward events at the cue phase, and no-reward and reward events at the task phase separately for the neutral and negative conditions.
Group analysis
Whole-brain voxelwise random-effects analyses were conducted using response estimates from individual-level analyses (restricted to gray-matter voxels) in AFNI. To probe reward processing during the cue phase, we used cue-phase responses (controlling for task-related activity) and ran a paired t-test to contrast reward and no-reward conditions. To probe the interaction between reward and distractor processing during the task phase, we used task-phase responses (controlling for cue-related activity) and ran a 2 Reward (reward, no-reward) × 2 Distractor (negative, neutral) repeated-measures ANOVA.
The alpha-level for voxelwise statistical analysis was determined by simulations using the 3dClustSim program. For these simulations, the smoothness of the data was estimated using 3dFWHMx program based on the residual time series from the individual-level voxelwise analysis. Taking into account the recent report of increased false-positive rates linked to the assumption of Gaussian spatial autocorrelation in fMRI data (Eklund et al., 2016), we used the new -acf (i.e. auto-correlation function) option recently added to the 3dFWHMx and 3dClustSim tools, which models spatial fMRI noise as a mixture of Gaussian plus mono-exponential distributions. This improvement was shown to control false positive rates around desired alpha level, especially with randomized fast event-related designs as employed in this study (Cox et al., 2017). Based on a voxel-level uncorrected P-value of 0.001, simulations indicated a minimum cluster extent of 48 voxels (2.0 × 2.0 × 2.0 mm) for a cluster-level corrected alpha of 0.05.
Relationship between cue-phase brain responses and behavior during the task phase
The only difference between the reward and no-reward conditions in our design was the type of advance cue employed (Figure 1). Thus, to investigate the potential link between brain responses at the cue phase and subsequent behavior during the task phase, we focused on the ventral striatum, a region consistently implicated in reward-related processing (Haber and Knutson, 2010; Bissonette et al., 2014). We defined bilateral ventral striatum regions of interest (ROIs) based on the structural atlas provided in the FSL package. First, we probed the relationship between cue-phase responses and an energizing effect of reward on behavior, as indexed by reward main effect scores. Next, we probed the relationship between cue-phase responses and selective effects of reward on behavior, as indexed by a reward by distractor interference scores.
Relationship between task-phase brain responses and behavior
To investigate the potential link between brain responses at the task phase (controlling for cue-related activity) and behavior, we ran a voxelwise robust skipped correlation analysis (across participants) between fMRI-based interaction scores at the task phase and RT-based interaction scores.
Functional connectivity analysis during cue phase
We investigated functional interactions between the ventral striatum and the rest of the brain by employing voxelwise functional connectivity analysis based on trial-by-trial responses. Similar to the so-called ‘beta series correlation method’ (Rissman et al., 2004), for each participant, we estimated trial-based responses at the cue phase (controlling for task-related activity) using the 3dLSS command in AFNI, which improves estimation of trial-based responses in fast-event related designs (Mumford et al., 2012).
To create representative ROI estimates, trial-based responses were averaged across voxels within the anatomically defined left and right ventral striatum ROI, separately. We then calculated the trial-by-trial robust skipped correlation between ventral striatum responses and the rest of the brain in a voxelwise fashion, separately for the reward and no-reward conditions. Correlations were contrasted between reward and no-reward conditions at the group level via a paired t-test using the 3dttest ++ program. Our approach is conceptually the same as psychophysiological interaction analysis (Friston et al., 1997), and shown to have better power to detect functional interactions in event-related designs (Cisler et al., 2014).
Results
Behavioral results
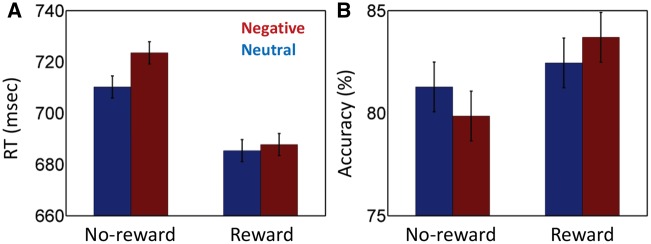
Mean RT data were evaluated according to a 2 Reward (reward, no-reward) × 2 Distractor (negative, neutral) repeated-measures ANOVA (Figure 3A). The main effect of Reward was significant (F1, 56 = 135.59, P = 0.000, η2P = 0.71). Mean RT was faster during the reward (686 ms) compared to the no-reward condition (716 ms), demonstrating the effectiveness of the reward manipulation. The main effect of Distractor was also significant (F1, 56 = 6.17, P = 0.016, η2P = 0.10), such that RTs during the negative condition (705 ms) were slower compared to the neutral condition (697 ms), revealing a small but consistent effect of the distractor stimulus. Critically, the Reward × Distractor interaction was significant (F1, 56 = 4.44, P = 0.040, η2P = 0.07). To further probe the two-way interaction, we ran two additional paired t-tests (negative vs neutral) for the reward and no-reward conditions, separately. We detected a robust negative interference effect during the no-reward condition (13 ms; t(56) = 2.98, P = 0.004, d = 0.39), but not during the reward condition (2 ms; t(56) = 0.66, P = 0.51, d = 0.09).
Fig. 3.
Behavioral results. (A) RT data. During no-reward trials, negative images slowed responses relative to neutral ones. This interference effect was reduced during the reward condition. (B) Accuracy data. During no-reward trials, negative images reduced accuracy relative to neutral ones. This effect was reduced during the reward condition. Error bars denote standard within-subject error term for interaction effects (Loftus and Masson, 1994).
Because, we observed a main effect of Reward such that the overall RT was faster during the reward condition, it is conceivable that the reduced negative interference during the reward condition was due to overall faster RTs (faster RTs would leave less room for interference). Therefore, we calculated a ratio-based index of interference (negative/neutral) separately for the reward and no-reward conditions. A comparison of the two via a paired t-test revealed a difference that is just outside the critical boundary (t(56) = 1.896, P = 0.06, d = 0.25), supporting the interpretation of reduced negative interference during the reward condition. Furthermore, the main effect of Reward and interaction scores were not strongly correlated across participants (robust skipped correlation: r = 0.17, P = 0.32) while the original and ratio-based indices of interaction scores were highly correlated across participants (robust skipped correlation: r = 0.99, P < 0.001), suggesting that the reduction of negative interference during reward was not simply due to overall faster RTs.
The 2 Reward × 2 Distractor repeated-measures ANOVA on mean accuracy rate data (Figure 3B) also revealed a main effect of Reward (F1, 56 = 17.71, P < 0.001, η2P = 0.24), such that accuracy was higher during reward (83.1%) compared to no-reward (80.6%). The main effect of Distractor was not significant (F1, 56 = 0.02, P = 0.895, η2P = 0.00). Finally, the Reward × Distractor interaction was just outside the critical decision boundary (F1, 56 = 3.62, P = 0.06, η2P = 0.06).
The RT and accuracy results taken together rule out the possibility of a speed-accuracy tradeoff, as participants were both more accurate and faster during the reward compared to no-reward condition. Further, faster RTs also rule out the possibility of cautious responding during reward trials, which could help reduce negative-picture interference. Overall, the behavioral results point to a more selective effect of reward on negative distractor processing.
Functional MRI results
Cue phase
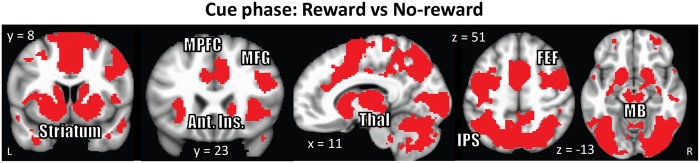
To investigate the impact of the reward processing, we contrasted cue-related responses (controlling for task-related activity) during reward vs no-reward conditions. This contrast revealed stronger responses during the reward condition in a large cluster that spanned multiple subcortical and cortical sites (Figure 4 and Table 1; in Tables 1–3, peak coordinates are presented for completeness and potential meta-analysis; with cluster-based thresholding, it is not possible to conclude that all the reported peaks were activated [see Woo et al., 2014]), including dorsal and ventral striatum (the latter including the accumbens), midbrain, thalamus, as well as fronto-parietal regions.
Fig. 4.
Cue phase functional MRI results displayed at an uncorrected P = 0.001 and 48-voxel cluster extent (cluster-level alpha of 0.05). Because cluster-extent based thresholding was used for multiple comparisons correction, voxels are displayed using a binary threshold. For further rationale about using binary maps in the context of cluster-based thresholding, see Woo et al, (2014). Increased responses were observed during processing of reward (vs no-reward) cue in subcortical regions and fronto-parietal regions. MPFC: medial prefrontal cortex, MFG: middle frontal gyrus, Ant. Ins: anterior insula, Thal: Thalamus, MB: midbrain, FEF: frontal eye-fields, IPS: intraparietal sulcus.
Table 1.
Clusters that survived multiple comparisons correction in voxelwise analysis at the cue phase (peak MNI coordinates, unstandardized effect size in terms of % signal change [ES = (CueREWARD – CueNO_REWARD)], t(56) values, and cluster size [k])
| Cluster | x | y | z | ES | T | k | |
|---|---|---|---|---|---|---|---|
| Cluster 1 (including fronto-parietal cortical regions and subcortical areas) | R/L | 36 | −88 | −8 | 0.10 | 10.03 | 51669 |
| Lateral orbitofrontal cortex | L | −22 | 46 | −16 | 0.04 | 5.29 | 96 |
| Frontal cluster (including middle frontal gyrus) | L | −26 | 46 | 18 | 0.05 | 8.06 | 1910 |
| Inferior temporal gyrus | L | −58 | −18 | −30 | 0.04 | 4.46 | 50 |
| Temporal pole | R | 50 | 18 | −20 | 0.04 | 6.24 | 155 |
| Temporal pole | L | −42 | 12 | −24 | 0.04 | 4.61 | 124 |
Peak coordinates are presented for completeness and potential meta-analysis; with cluster-based thresholding, it is not possible to conclude that all the reported peaks were activated (see Woo et al., 2014).
Task phase
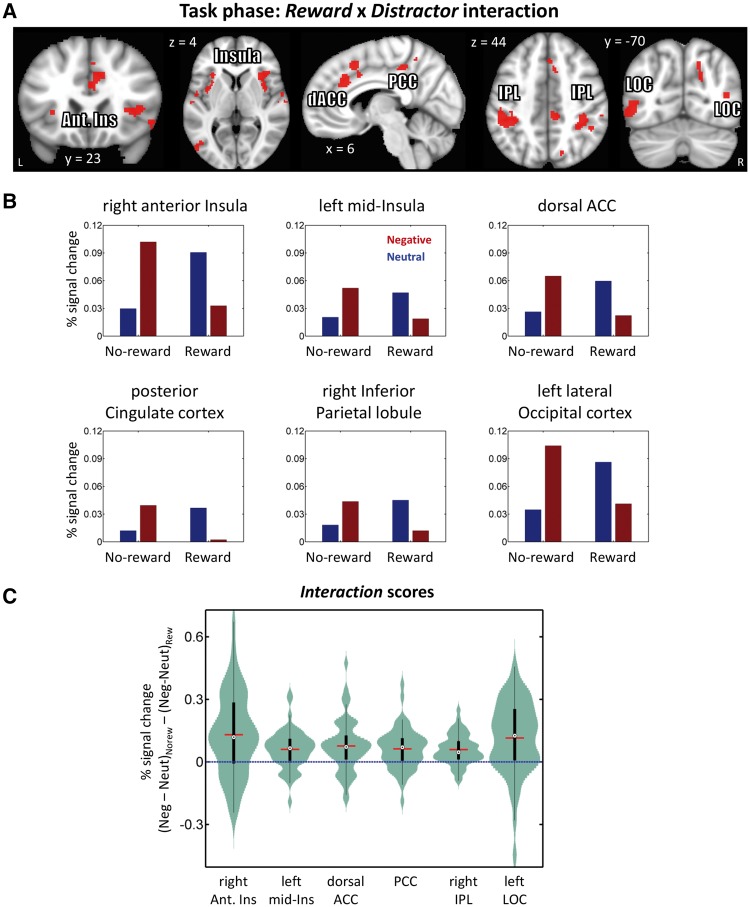
The main focus of this study was to investigate the influence of potential reward on negative interference, which was examined via the Reward × Distractor interaction on task-phase responses (controlling for cue-related activity). We observed significant interaction effects in several activation clusters, including in medial prefrontal cortex (spanning dorsal anterior cingulate cortex and supplementary motor area), posterior cingulate cortex, right anterior/mid-insula, right inferior parietal lobule, left lateral occipital cortex (at inferior and superior locations) and right lateral occipital cortex (at a superior location), as well as a large left fronto-parietal cluster (spanning inferior parietal lobule and anterior/mid-insula) (Figure 5 and Table 2). Paralleling the behavioral interaction pattern, in the clusters that exhibited an interaction pattern, stronger negative (vs neutral) responses during the no-reward condition were reduced during the reward condition. In the control analysis where cue and task phase regressors were modeled simultaneously (see Methods), a similar interaction pattern was observed in the clusters mentioned above except that medial prefrontal cortex (35 voxels), posterior cingulate cortex (35 voxels) and bilateral lateral posterior occipital cortex (left inferior: 36 voxels; left superior: 15 voxels; right superior: 10 voxels) did not survive the multiple comparisons correction.
Fig. 5.
Task phase functional MRI results. (A) Clusters exhibiting significant Reward (reward, no-reward) × Distractor (neutral, negative) interactions displayed at an uncorrected P = 0.001 and 48-voxel cluster extent (cluster-level alpha of 0.05). Because cluster-extent based thresholding was used for multiple comparisons correction, voxels are displayed using a binary threshold. For further rationale about using binary maps in the context of cluster-based thresholding, see Woo et al. (2014). (B) Average responses within clusters that exhibited interaction effects. As some clusters spanned large sectors, for illustration purposes, clusters were selected at an uncorrected P =0.0005 and 34-voxel cluster extent (cluster-level alpha of 0.05). To avoid circularity, error bars are not plotted. (C) Distributions of within-subject interaction scores in units of % signal change. The box plots within the violins indicate the interquartile range (first quartile to third quartile), red lines show the mean values, and black dots inside white circles show median values. Ant. Ins: anterior insula, IPL: inferior parietal lobule, dACC: dorsal anterior cingulate cortex, PCC: posterior cingulate cortex, LOC: lateral occipital cortex.
Table 2.
Clusters that survived multiple comparisons correction in voxelwise analysis at the task phase (peak MNI coordinates, unstandardized effect size in terms of % signal change [ES], F(1,56) values, and cluster size [k])
| Cluster | x | y | z | ES | F | k | |
|---|---|---|---|---|---|---|---|
|
Reward × Distractor | |||||||
| ES = ([Negative − Neutral]NO_REWARD – [Negative – Neutral]REWARD) | |||||||
| Medial prefrontal cortex (including dorsal anterior cingulate cortex and supplementary motor area) | R | 6 | 28 | 36 | 0.07 | 21.17 | 258 |
| Inferior parietal lobule | R | 34 | −34 | 52 | 0.06 | 34.67 | 350 |
| Fronto-parietal cluster (including inferior parietal lobule and anterior/mid insula) | L | −46 | −38 | 44 | 0.08 | 30.67 | 1176 |
| anterior/mid insula | R | 42 | 24 | 6 | 0.07 | 22.95 | 360 |
| Posterior cingulate cortex | R | 8 | −36 | 46 | 0.07 | 26.09 | 88 |
| Lateral occipital cortex (inferior) | L | −52 | −70 | −6 | 0.15 | 23.18 | 224 |
| Lateral occipital cortex (superior) | L | −44 | −82 | 18 | 0.09 | 23.41 | 86 |
| Lateral occipital cortex (superior) | R | 42 | −72 | 12 | 0.06 | 24.13 | 77 |
| Supramarginal gyrus (inferior) | R | 62 | −24 | 26 | 0.08 | 18.55 | 72 |
| Supramarginal gyrus (superior) | R | 62 | −34 | 48 | 0.10 | 16.86 | 79 |
| Superior temporal gyrus | R | 64 | −2 | −4 | 0.14 | 20.81 | 187 |
| Precuneus | R | 18 | −68 | 32 | 0.06 | 18.29 | 56 |
| Main effect of Reward | |||||||
|
ES = ([Negative + Neutral]REWARD – [Negative + Neutral]NO_REWARD) | |||||||
| Postcentral gyrus | R | −66 | −22 | 18 | −0.03 | 20.65 | 103 |
| Main effect of Distractor | |||||||
|
ES = ([Negative − Neutral]REWARD + [Negative – Neutral]NO_REWARD) | |||||||
| Ventral visual cortex (including fusiform gyrus) | R | 38 | −68 | −12 | 0.08 | 76.64 | 1682 |
| Ventral visual cortex (including fusiform gyrus) | L | −36 | −80 | −10 | 0.06 | 73.58 | 1695 |
| Amygdala | R | 24 | −4 | −14 | 0.06 | 32.99 | 84 |
| Amygdala | L | −22 | −6 | −12 | 0.05 | 26.05 | 48 |
| lateral orbitofrontal cortex | R | 28 | 32 | −12 | 0.05 | 35.62 | 97 |
| lateral orbitofrontal cortex | L | −38 | 34 | −12 | 0.07 | 28.58 | 166 |
| Anterior insula | L | −40 | 26 | 4 | 0.03 | 24.98 | 66 |
| Inferior frontal gyrus | R | 44 | 12 | 26 | 0.06 | 20.61 | 86 |
| Superior parietal/occipital cortex | R | 26 | −60 | 52 | 0.03 | 28.89 | 153 |
| Superior parietal/occipital cortex | L | −22 | −66 | 40 | 0.05 | 24.96 | 61 |
| Ventral striatum | R | 16 | 18 | −4 | −0.03 | 17.18 | 31U |
| Ventral striatum | L | −6 | 16 | −6 | −0.04 | 21.09 | 133 |
| Lateral occipital cortex (superior) | R | 44 | −70 | 36 | −0.03 | 28.48 | 315 |
| Lateral occipital cortex (superior) | L | −42 | −60 | 50 | −0.03 | 16.81 | 92 |
| Middle frontal gyrus | R | 30 | 18 | 46 | −0.03 | 24.35 | 402 |
| Middle frontal gyrus | L | −34 | 16 | 50 | −0.03 | 21.36 | 103 |
| Supramarginal gyrus (posterior) | R | 48 | −42 | 40 | −0.02 | 23.76 | 118 |
| Occipital cluster (including lingual gyrus) | R | 12 | −76 | −2 | −0.04 | 32.74 | 731 |
| Superior parietal lobule | L | −26 | −48 | 70 | −0.04 | 25.83 | 276 |
| Precuneus | R | 14 | −54 | 8 | −0.07 | 21.37 | 69 |
| Precuneus | L | −6 | −66 | 52 | −0.03 | 18.53 | 56 |
Peak coordinates are presented for completeness and potential meta-analysis; with cluster-based thresholding, it is not possible to conclude that all the reported peaks were activated (see Woo et al., 2014).
Cluster did not survive correction for multiple comparisons.
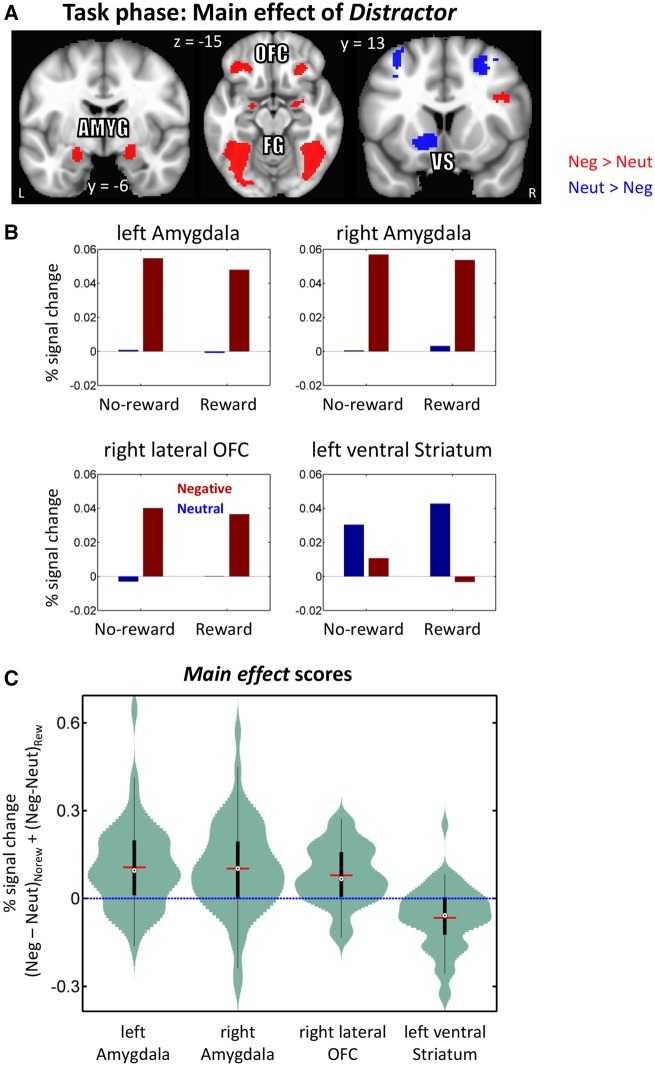
We observed a main effect of Distractor in clusters including the bilateral amygdala, bilateral ventral visual cortex/fusiform gyrus, bilateral lateral orbitofrontal cortex and left ventral striatum (a similar pattern was seen in right ventral striatum at a slightly lower threshold; Figure 6 and Table 2). In the amygdala, ventral visual cortex/fusiform gyrus and orbitofrontal cortex, stronger responses were observed during the negative compared to the neutral condition. However, in the ventral striatum we observed the converse pattern, where responses were weaker during the negative compared to the neutral condition.
Fig. 6.
Task phase functional MRI results displayed at an uncorrected P = 0.001 and 48-voxel cluster extent (cluster-level alpha of 0.05). Because cluster-extent based thresholding was used for multiple comparisons correction, voxels are displayed using a binary threshold. For further rationale about using binary maps in the context of cluster-based thresholding, see Woo et al. (2014). (A) Clusters exhibiting significant main effect of Distractor type (neutral, negative). (B) Main-effect response pattern from a subset of regions. To avoid circularity, error bars are not plotted. (C) Distributions of within-subject main effect of Distractor scores in units of % signal change. The box plots within the violins indicate the interquartile range (first quartile to third quartile), red lines show the mean values, and black dots inside white circles show median values. Amyg: amygdala, OFC: orbital frontal cortex, VS: ventral striatum, FG: fusiform gyrus.
Relationship between cue-related responses and behavior
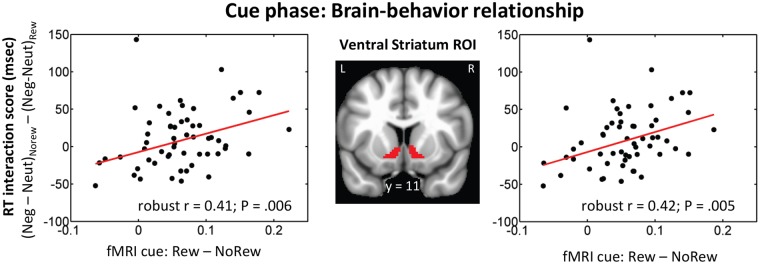
To probe the link between cue-related responses and subsequent behavior, we focused on the ventral striatum, a region that has been consistently implicated in reward-related processing. We evaluated the relationship between cue-phase responses and behavioral reward main effects, which would reflect an unspecific reward effect. The robust correlation analysis revealed a positive relationship between reward (vs no-reward) responses to the cue and behavioral RT scores in the right ventral striatum ROI (robust skipped correlation r = −0.31, P = 0.02) and was just outside the critical decision boundary in the left ventral striatum ROI (robust skipped correlation r = −0.25, P = 0.06), indicating that participants with stronger reward-related cue responses were behaviorally faster during the reward condition. We also evaluated the relationship between cue-phase responses and behavioral interaction scores which would reflect a more specific effect of reward on behavior. In both left and right ventral striatum anatomical ROIs, across-subject robust correlation analysis revealed a significant positive relationship between reward (vs no-reward) responses to the cue and behavioral RT interaction scores (Figure 7). This linear relationship indicates that participants with stronger reward-related responses in the ventral striatum during the cue phase were more successful at reducing the impact of negative images on task performance.
Fig. 7.
Brain-behavior relationship. A positive linear relationship was observed between reward (vs no-reward) cue responses and behavioral RT interaction scores in the right and left ventral striatum ROIs.
Relationship between task-related responses and behavior
A whole-brain voxelwise robust correlation analysis between task phase-responses and behavior did not detect significant linear relationships.
Functional connectivity during cue phase
In line with previous studies investigating the impact of cues signaling reward (Harsay et al., 2011; Padmala and Pessoa, 2011), we anticipated stronger functional connectivity between the ventral striatum and cortical sites involved in attention and goal-directed processing during reward trials. To probe functional interactions, we determined trial-based functional connectivity of the ventral striatum with voxels across the brain. This analysis revealed stronger functional coupling during reward (vs no-reward) with cortical sites, including parietal cortex and sites consistent with the frontal eye-fields (Figure 8 and Table 3).
Fig. 8.
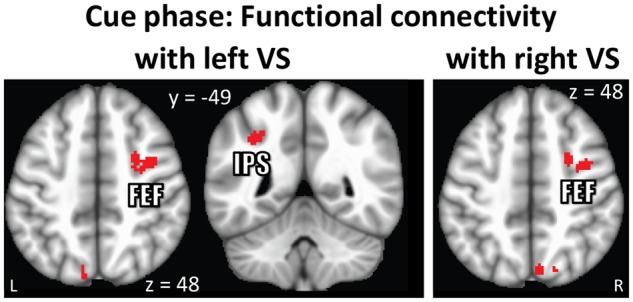
Cue phase functional connectivity results displayed at an uncorrected P = 0.001 and 48-voxel cluster extent (cluster-level alpha of 0.05). Because cluster-extent based thresholding was used for multiple comparisons correction, voxels are displayed using a binary threshold. For further rationale about using binary maps in the context of cluster-based thresholding, see Woo et al. (2014). (A) Stronger functional coupling was observed between ventral striatum and fronto-parietal regions during the processing of reward relative to no-reward cues. VS: ventral striatum, FEF: frontal eye-fields, IPS: intraparietal sulcus.
Table 3.
Clusters that survived multiple comparisons correction in voxelwise functional connectivity analysis with ventral striatum at cue phase (peak MNI coordinates, unstandardized effect size in terms of differential correlation [ES = (rREWARD – rNO_REWARD)], t(56) values, and cluster size [k])
| Cluster | x | y | z | ES | t | k | |
|---|---|---|---|---|---|---|---|
| With left ventral striatum ROI | |||||||
| Frontal eye fields | R | 38 | −2 | 46 | 0.15 | 6.27 | 345 |
| Intraparietal sulcus | L | −32 | −48 | 42 | 0.11 | 4.79 | 60 |
| Supplementary motor cortex | R/L | −2 | −4 | 68 | 0.11 | 5.05 | 173 |
| Lateral occipital cortex (superior) | L | −8 | −80 | 50 | 0.12 | 5.50 | 76 |
| With right ventral striatum ROI | |||||||
| Frontal eye fields | R | 26 | 2 | 48 | 0.11 | 5.16 | 118 |
| Precuneus | R | 6 | −72 | 50 | 0.11 | 4.74 | 50 |
Peak coordinates are presented for completeness and potential meta-analysis; with cluster-based thresholding, it is not possible to conclude that all the reported peaks were activated (see Woo et al., 2014).
Discussion
We investigated the interaction between potential reward and negative distractor processing in the human brain. Behaviorally, the prospect of reward signaled by an advance cue reduced the influence of negative distractors during a subsequent perceptual discrimination task. In terms of brain responses, interactions paralleling behavior were observed across multiple sites, including the anterior insula and dorsal anterior cingulate cortex. Notably, in some brain regions, including the amygdala and fusiform gyrus, we did not detect the influence of potential reward on distractor processing. Together, our fMRI findings revealed evidence for both interaction and independence between potential reward driven top-down attentional control mechanisms and negative-distractor processing.
Based on observed patterns of behavior, a few studies have proposed that aversive distractor processing is immune to potential reward, at least under certain conditions (Kaltwasser et al., 2013; Wei and Kang, 2014). Kaltwasser et al. (2013) did not detect an interaction between reward expectancy and task-irrelevant valence in a word categorization task, and suggested that the processing of emotionally valenced words is immune to a reward manipulation. Wei and Kang (2014) did not detect an interaction between the valence of face stimuli and potential reward when faces were task irrelevant like in the present study; they did observe an interaction when the valence of the faces was relevant to the task. The behavioral findings of the present study revealed a potential reward by distractor type interaction that replicated our previous behavior-only study (Padmala and Pessoa, 2014). More broadly, the interaction pattern observed here is consistent with other behavioral findings of enhanced processing of goal-relevant information in the presence of threat-related distractors (Vogt et al., 2013; Vromen et al., 2015).
In terms of brain signals, during the cue phase, consistent with the previous literature from both human and animal studies (Haber and Knutson, 2010; Liu et al., 2011), we observed stronger responses during reward trials in a large cluster that included subcortical regions involved in reward-related processing, including the striatum and midbrain, as well as parietal and frontal cortical sites important for attention and executive function (Padmala and Pessoa, 2011; Krebs et al., 2012). In addition, we detected increased functional connectivity between the ventral striatum and a subset of the fronto-parietal regions (Harsay et al., 2011; Padmala and Pessoa, 2011). As the only difference between the reward and no-reward conditions was the type of cue employed, these results suggest that the processing of the reward relative to the no-reward cue led to the enhancement of proactive executive control mechanisms (Chiew and Braver, 2013) that enabled participants to better handle the impact of the negative distractors.
Consistent with this notion, across participants, we observed a significant link between cue-related response strength in the ventral striatum/accumbens and behavioral interaction scores during the subsequent task phase. Participants with stronger responses to reward cues in the ventral striatum exhibited a greater benefit of reward in reducing negative-picture interference. This brain-behavior relationship is in line with proposals that ascribe a role for the ventral striatum in driving the redistribution of processing resources (Sarter et al., 2006; Salamone et al., 2009), which can lead to more selective effects of reward on behavioral performance (see also Pessoa, 2013). In this context, cue-related activity in the ventral striatum might have reflected processes engaged to handle challenging situations (in this case, handling aversive distractors). As observed here, these processes might have involved the up-regulation of attentional control regions in frontal and parietal cortex, as reflected in the enhanced functional connectivity observed during reward cues (see also Harsay et al., 2011; Padmala and Pessoa, 2011). But note that an association between cue responses and an unspecific effect of reward on behavior was also detected, consistent with the idea that reward cues also served an energizing function.
During the task phase, we detected significant interaction effects in frontal and parietal brain regions, including the anterior and mid-insula, medial prefrontal cortex, and inferior parietal lobule. Paralleling the behavioral data, in all these sites, differential responses (negative minus neutral) were reduced during the reward condition. The finding in the anterior insula is noteworthy given its involvement in negative processing (Phillips et al., 2003; Craig, 2009), including the processing of mutilation images (Wright et al., 2004; Sarinopoulos et al., 2010). Another noteworthy site of interaction was in a medial prefrontal cortex cluster that included the dorsal anterior cingulate cortex. Based on the review of human and animal literatures, Etkin et al. (2011) suggested that dorsal aspects of the anterior cingulate cortex are involved in emotional appraisal/expression. Our findings mesh well with this proposal as, in line with behavior, the differential response (negative minus neutral) to distractors was positive during the control condition (no-reward), but flipped sign during the reward condition. Thus, in the context of our task, the dorsal anterior cingulate exhibited a profile consistent with emotional reactivity (see also Etkin et al., 2015) that was influenced by motivational processing.
We also observed a significant interaction effect during the task phase in the lateral occipital cortex where negative (vs neutral) responses were decreased during the reward condition. Intriguingly, in a meta-analysis of emotional perception studies (Sabatinelli et al., 2011), the lateral occipital cortex was found to be engaged during the processing of complex emotional images such as mutilated bodies. If, in our study, the lateral occipital cortex activation reflected engagement by aversive pictures, the interaction pattern would be consistent with the idea that potential reward decreased the impact of such stimuli.
An important question concerns the pattern of responses in the amygdala. Given that negative-distractor interference was essentially eliminated behaviorally, one could have anticipated that differential responses (negative greater than neutral) observed during no-reward would be eliminated (or at least reduced) during the reward condition. However, only a main effect of distractor type in the anticipated direction (negative greater than neutral) was observed, not an interaction. Thus, in our task, the prospect of reward did not appreciably influence how the amygdala responded to aversive images. This overall pattern of response should be contrasted to the one observed during demanding attentional manipulations; when attention is robustly consumed elsewhere, the impact of unattended emotional stimuli is largely decreased or even eliminated (Pessoa, 2002; Mitchell et al., 2007). Such findings have been interpreted as indicating that the processing of emotional stimuli is not automatic, and that they can be filtered out during strong attentional manipulations. In the present study, we observed interaction effects across several regions, including the anterior insula, dorsal anterior cingulate, and inferior parietal lobule, but not in the amygdala. Thus, advance reward did not appear to lead to the filtering out of emotional distractors (see Vuilleumier, 2005). However, the deleterious impact of negative images was eliminated behaviorally. It is thus likely that the regions exhibiting the reward by emotion interaction play a role in reducing the impact of negative distractors. It is worth pointing out that the pattern of responses observed in the amygdala was similar to the one we saw when reward was manipulated in a reactive fashion, that is, where certain stimuli were linked to reward and no advance reward cues were used (Hu et al., 2013; see also Wittmann et al., 2008); in such cases, the impact of negative stimuli in the amygdala was not counteracted by reward.
Previous studies have shown that motivation enhances attention when participants are explicitly informed of the possibility of reward (Engelmann et al., 2009; Hubner and Schlosser, 2010; Krebs et al., 2012). In a previous study (Padmala and Pessoa, 2011), participants were informed of the possibility of reward by a cue stimulus that preceded the target phase during which a Stroop-like interference stimulus was displayed. Behaviorally, participants exhibited reduced conflict during the reward vs no-reward condition. Brain imaging results revealed that a group of subcortical and fronto-parietal regions was robustly influenced by reward at cue processing and, importantly, that increased cue-related responses in fronto-parietal regions important for attention were correlated with reduced conflict-related signals during the upcoming target phase. Together, our findings were consistent with a model in which motivationally salient cues are employed to upregulate top-down control processes that bias the selection of visual information, thereby leading to more efficient stimulus processing during conflict conditions.
In the current study, we observed a similar behavioral pattern of reduced interference (here with negative distractors) as in our previous study (Padmala and Pessoa, 2011). However, the absence of an interaction effect in the amygdala and fusiform gyrus suggests a modified interpretation of our results. Given the intermediate attentional demands of the bar task, negative images were registered by the amygdala (and lateral orbitofrontal cortex; see Figure 6), impacting processing along visual cortex (Vuilleumier et al., 2004; Hadj-Bouziane et al., 2012). However, enhanced attentional control mechanisms during the reward condition enabled participants to reduce the deleterious impact of negative images. Enhanced attentional control appeared to be linked to interactions between the ventral striatum and fronto-parietal regions important for executive control (in and around the frontal eye fields, and posterior intraparietal sulcus). Furthermore, as previously stated, it is likely that the regions exhibiting the reward by emotion interaction play a more direct role than regions possibly involved in registering the stimulus itself (for instance, amygdala and fusiform gyrus). We note, however, that in regions exhibiting the reward by emotion interaction, evidence for a brain-behavior relationship was not detected in the present study.
In conclusion, our study demonstrated the influence of performance-based monetary rewards on aversive processing. In terms of behavior, negative interference scores were reduced during the reward condition and a parallel interaction pattern was observed in several brain regions, including the anterior insula, dorsal anterior cingulate, and inferior parietal lobule. During reward cue processing, stronger responses were observed in subcortical regions important for reward processing, as well as fronto-parietal regions important for cognitive control. Notably, stronger reward-cue responses in the ventral striatum were associated with reduced behavioral negative interference during the subsequent task phase. Together, our study refines our understanding of the brain mechanisms underlying the influence of potential reward on the processing of negative distractors in the human brain.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
Supplementary Material
Acknowledgements
We thank the anonymous reviewers for valuable feedback on earlier versions of the manuscript. We also thank Mirtes Pereira for sharing stimuli, Jason Smith and Joshua Kinnison for help with processing scripts, Brenton McMenamin for helpful discussions, and Nicole Friedman for help with subject recruitment. The authors also acknowledge the Behavioral and Social Sciences College, University of Maryland, high performance computing resources (http://bsos.umd.edu/oacs/bsos-high-performance) made available for conducting the research reported in this paper.
Funding
The Support for this work was provided in part by the National Institute of Mental Health (R01 MH071589 to Luiz Pessoa) and a National Science Foundation Graduate Research Fellowship to Srikanth Padmala.
References
- Bissonette G.B., Gentry R.N., Padmala S., Pessoa L., Roesch M.R. (2014). Impact of appetitive and aversive outcomes on brain responses: linking the animal and human literatures. Frontiers in Systems Neuroscience, 8, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler C.N., Hopf J.M., Stoppel C.M., Krebs R.M. (2012). Motivating inhibition: reward prospect speeds up response cancellation. Cognition, 125, 498–503. [DOI] [PubMed] [Google Scholar]
- Botvinick M., Braver T. (2015). Motivation and cognitive control: from behavior to neural mechanism. Psychology, 66, 83. [DOI] [PubMed] [Google Scholar]
- Braem S., Verguts T., Roggeman C., Notebaert W. (2012). Reward modulates adaptations to conflict. Cognition, 125, 324–32. [DOI] [PubMed] [Google Scholar]
- Chiew K.S., Braver T.S. (2013). Temporal dynamics of motivation-cognitive control interactions revealed by high-resolution pupillometry. Frontiers in Psychology, 4, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M., Bush K., Steele J.S. (2014). A comparison of statistical methods for detecting context-modulated functional connectivity in fMRI. Neuroimage, 84, 1042–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. (1997). Parametric analysis of fMRI data using linear systems methods. Neuroimage, 6, 93–103. [DOI] [PubMed] [Google Scholar]
- Cox, R.W., Chen, G., Glen, D.R., Reynolds, R.C., Taylor, P.A. (2017). FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connectivity, 7,152–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. (2009). How do you feel-now? the anterior insula and human awareness. Nature Reviews Neuroscience, 10, 59–70. [DOI] [PubMed] [Google Scholar]
- Dillon D.G., Rosso I.M., Pechtel P., Killgore W.D., Rauch S.L., Pizzagalli D.A. (2014). Peril and pleasure: an rdoc-inspired examination of threat responses and reward processing in anxiety and depression. Depression and Anxiety, 31, 233–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. (2016) Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences USA, 113(28), 7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann J.B., Damaraju E.C., Padmala S., Pessoa L. (2009). Combined effects of attention and motivation on visual task performance: transient and sustained motivational effects. Frontiers in Human Neuroscience, 3, 4. doi:10.3389/neuro.3309.3004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Buchel C., Gross J.J. (2015). The neural bases of emotion regulation. Nature Review Neuroscience, 16, 693–700. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. (1997). Psychophysiological and modulatory interactions in neuroimaging. Neuroimage, 6, 218–29. [DOI] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology, 35, 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadj-Bouziane F., Liu N., Bell A.H., et al. (2012). Amygdala lesions disrupt modulation of functional MRI activity evoked by facial expression in the monkey inferior temporal cortex. Proceedings of the National Academy of Sciences USA, 109, E3640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay H.A., Cohen M.X., Oosterhof N.N., Forstmann B.U., Mars R.B., Ridderinkhof K.R. (2011). Functional connectivity of the striatum links motivation to action control in humans. Journal of Neuroscience, 31, 10701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, K., Padmala, S., Pessoa, L. (2013). Interactions between reward and threat during visual processing. Neuropsychologia, 51, 1763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner R., Schlosser J. (2010). Monetary reward increases attentional effort in the flanker task. Psychonomic Bulletin and Review, 17, 821–6. [DOI] [PubMed] [Google Scholar]
- Kaltwasser L., Ries S., Sommer W., Knight R., Willems R.M. (2013). Independence of valence and reward in emotional word processing: electrophysiological evidence. Frontiers in Psychology, 4, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs R.M., Boehler C.N., Roberts K.C., Song A.W., Woldorff M.G. (2012). The involvement of the dopaminergic midbrain and cortico-striatal-thalamic circuits in the integration of reward prospect and attentional task demands. Cerebral Cortex, 22, 607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Berghorst L.H., Nickerson L.D., et al. (2014). Differential effects of acute stress on anticipatory and consummatory phases of reward processing. Neuroscience 266, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, P.J., Bradley, M.M., Cuthbert, B.N. (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida, Gainesville, FL. [Google Scholar]
- Liu X., Hairston J., Schrier M., Fan J. (2011). Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 35, 1219–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus G.R., Masson M.E. (1994). Using confidence intervals in within-subject designs. Psychonomic Bulletin and Review, 1, 476–90. [DOI] [PubMed] [Google Scholar]
- Mitchell D.G., Nakic M., Fridberg D., Kamel N., Pine D.S., Blair R.J. (2007). The impact of processing load on emotion. Neuroimage, 34, 1299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocaiber I., Sanchez T.A., Pereira M.G., et al. (2011). Antecedent descriptions change brain reactivity to emotional stimuli: a functional magnetic resonance imaging study of an extrinsic and incidental reappraisal strategy. Neuroscience, 193, 241–8. [DOI] [PubMed] [Google Scholar]
- Mumford J.A., Turner B.O., Ashby F.G., Poldrack R.A. (2012). Deconvolving BOLD activation in event-related designs for multivoxel pattern classification analyses. Neuroimage, 59, 2636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossewaarde L., Qin S., Van Marle H.J.F., van Wingen G.A., Fernández G., Hermans E.J. (2011). Stress-induced reduction in reward-related prefrontal cortex function. Neuroimage, 55, 345–52. [DOI] [PubMed] [Google Scholar]
- Padmala S., Pessoa L. (2011). Reward reduces conflict by enhancing attentional control and biasing visual cortical processing. Journal of Cognitive Neuroscience, 23, 3419–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmala S., Pessoa L. (2014). Motivation versus aversive processing during perception. Emotion, 14, 450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Q., Kahnt T., Rieskamp J., Heekeren H.R. (2011). Neurobiology of value integration: when value impacts valuation. Journal of Neuroscience, 31, 9307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. (2002). Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences USA, 99, 11458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. (2013) The Cognitive-Emotional Brain: From Interactions to Integration. Cambridge: MIT Press. [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. (2003). Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry, 54, 504–14. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A. (2014). Depression, stress, and anhedonia: toward a synthesis and integrated model. Annual Review of Clinical Psychology, 10, 393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli A.J., Lewis A.H., Delgado M.R. (2012). Acute stress influences neural circuits of reward processing. Frontiers in Neuroscience, 6, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J., Gazzaley A., D'Esposito M. (2004). Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage, 23, 752–63. [DOI] [PubMed] [Google Scholar]
- Ruge H., Goschke T., Braver T.S. (2009). Separating event-related BOLD components within trials: the partial-trial design revisited. Neuroimage, 47, 501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D., Fortune E.E., Li Q., et al. (2011). Emotional perception: meta-analyses of face and natural scene processing. Neuroimage, 54, 2524–33. [DOI] [PubMed] [Google Scholar]
- Salamone J.D., Correa M., Farrar A.M., Nunes E.J., Pardo M. (2009). Dopamine, behavioral economics, and effort. Frontiers in Behavioral Neuroscience, 3, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarinopoulos I., Grupe D.W., Mackiewicz K.L., et al. (2010). Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cerebral Cortex, 20, 929–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M., Gehring W.J., Kozak R. (2006). More attention must be paid: the neurobiology of attentional effort. Brain Research Reviews, 51, 145–60. [DOI] [PubMed] [Google Scholar]
- Savine A.C., Beck S.M., Edwards B.G., Chiew K.S., Braver T.S. (2010). Enhancement of cognitive control by approach and avoidance motivational states. Cognition and Emotion, 24, 338–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences J.T. (2004). A comparison of methods for characterizing the event-related BOLD timeseries in rapid fMRI. Neuroimage, 21, 1690–700. [DOI] [PubMed] [Google Scholar]
- Talmi D., Dayan P., Kiebel S.J., Frith C.D., Dolan R.J. (2009). How humans integrate the prospects of pain and reward during choice. Journal of Neuroscience, 29, 14617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt J., De Houwer J., Crombez G., Van Damme S. (2013). Competing for attentional priority: temporary goals versus threats. Emotion, 13, 587–98. [DOI] [PubMed] [Google Scholar]
- Vromen J.M., Lipp O.V., Remington R.W. (2015). The spider does not always win the fight for attention: disengagement from threat is modulated by goal set. Cogn Emot, 29, 1185–96. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. (2005). How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences, 9, 585–94. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Richardson M.P., Armony J.L., Driver J., Dolan R.J. (2004). Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience, 7, 1271–8. [DOI] [PubMed] [Google Scholar]
- Wei P., Kang G. (2014). Task relevance regulates the interaction between reward expectation and emotion. Experimental Brain Research, 232, 1783–91. [DOI] [PubMed] [Google Scholar]
- Wei P., Wang D., Ji L. (2016). Reward expectation regulates brain responses to task-relevant and task-irrelevant emotional words: ERP evidence. Social Cognitive and Affective Neuroscience, 11, 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann B.C., Schiltz K., Boehler C.N., Düzel E. (2008). Mesolimbic interaction of emotional valence and reward improves memory formation. Neuropsychologia, 46, 1000–8. [DOI] [PubMed] [Google Scholar]
- Woo C.W., Krishnan A., Wager T.D. (2014). Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage, 91, 412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P., He G., Shapira N.A., Goodman W.K., Liu Y. (2004). Disgust and the insula: fMRI responses to pictures of mutilation and contamination. Neuroreport, 15, 2347–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.