Abstract
Background:
Wound healing is a complex biological process. Some injuries lead to chronic nonhealing ulcers, and healing process is a challenge to both the patient and the medical team. We still look forward an appropriate wound dressing.
Materials and Methods:
In this study, starch-based nanocomposite hydrogel scaffolds reinforced by zeolite nanoparticles (nZ) were prepared for wound dressing. In addition, a herbal drug (chamomile extract) was added into the matrix to accelerate healing process. To estimate the cytocompatibility of hydrogel dressings, fibroblast mouse cells (L929) were cultured on scaffolds. Then, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium-bromide assay test and interaction of cells and scaffolds were evaluated. For evaluating healing process, 48 male rats were randomly divided into four groups of four animals each (16 rats at each step). The ulcers of the first group were treated with the same size of pure hydrogels. The second group received a bandage with the same size of hydrogel/extract/4 wt% nZ (hydrogel NZE). The third group was treated with chamomile extract, and the fourth group was considered as control without taking any medicament. Finally, the dressings were applied on the chronic refractory ulcers of five patients.
Results:
After successful surface morphology and cytocompatibility tests, the animal study was carried out. There was a significant difference between starch/extract/4 wt% nZ and other groups on wound size decrement after day 7 (P < 0.05). At the clinical pilot study step, the refractory ulcers of all five patients were healed without any hypersensitivity reaction.
Conclusion:
Starch-based hydrogel/zeolite dressings may be safe and effective for chronic refractory ulcers.
Keywords: Chamomile, hydrogel, nanocomposite, ulcer, wound, zeolite
INTRODUCTION
The skin provides a natural barrier against most potential pathogens and other material from the environment. However, skin injuries occur frequently in routine life. In wounding, epidermis was removed and the connective tissue becomes exposed. Wound healing takes place immediately after the injury as a natural survival mechanism.[1,2]
Wound healing is a complex biological process; cells such as platelets, neutrophils, monocytes/macrophages, fibroblasts, endothelial cells, along with extracellular matrix components, such as fibronectin, collagens, glycosaminoglycans, and proteoglycans are involved in the healing process of damaged tissues. There are three overlapping stages in the wound-healing process: inflammation, proliferation, and remodeling, followed by scar maturation.[3] After the injury, wound dressing has an important role in urgent wound treatment and can significantly accelerate the early and late stages of skin regeneration. Alternative wound dressings have been developed and suggested over the past decades such as bandages, hydrogels, foams, nanofibers, sponges, and films. However, the appropriate wound dressing is still awaited in the medicine. Most research focuses on the prevention of infection and the wound healing effects of wound dressings.[4]
Therefore, effective wound coverage will create great benefits regarding the duration of admission, healing time, and quality of patients’ lives. Hydrogels are suitable implements to absorb wound exudates, protect the wound against infection, and provide a wet environment to accelerate the wound healing.[5] Many studies have reported the antibacterial and antimetastatic effects of zeolite in wound healing. In addition, it is a powerful natural antioxidant and immunostimulator.[6] For centuries, chamomile with anti-inflammatory, antimicrobial, and antioxidant effects has been applied as a medicinal plant, mostly for gastrointestinal ailments and skin injuries and problems. It is used in wound care, where its effectiveness has been reported in healing burn and episiotomy wounds.[7,8] Therefore, in this study, starch-based nanocomposite hydrogel scaffolds reinforced by zeolite nanoparticles (nZ) and loaded by a herbal drug (chamomile extract) were prepared for wound dressing to accelerate healing process.
MATERIALS AND METHODS
Materials
Modified starch and glycerol were bought from Merck Company, Germany and nZ with average 20–90 nm in diameter, density 3:0 g/ml were purchased from Berkeley Advanced Biomaterials, Inc. CA, USA. Chamomile extract was provided from the Institute of traditional medicine and herbal plants of Iran (Esfahan, Iran).
Hydrogel preparation
The starch/nZ composites films were prepared by a two-step procedure based on the method described by Tang et al.[9,10] First, a mixture of 64 wt% corn starch and nZ (at 0, 1, 2, 3, and 4 wt% nanoparticles/starch ratio), 15 wt% glycerol, 19 wt% water, and 2 wt% chamomile extract were extruded by a twin screw extruder (C. W. Brabender, Model 2802) at a screw speed of 200 rpm. Then, the extruded mixture was grounded by a Willy mill and an ultra-mill making a dry powder. At the next step, a solution contained 96 wt% deionized water and 4 wt% powders was prepared and mixed to obtain a homogenous mixture. Afterward, it was heated at 95°C for 10 min with constant stirring. Finally, the gelatinized solution was casted into sterile plates and degassed at 65°C for 2 h under vacuum. After casting, the plates were dried at 23° C and 50% relative humidity for 2 days. Then the transparency index and moisture absorption of scaffolds were assessed using the method of Nasri-Nasrabadi et al.[11]
Drug release measurement
To test the drug release behavior of hydrogels at various nanoparticle contents, the samples were cut with the dimension of 30 mm × 10 mm and immersed in the phosphate buffered saline (PBS) solution containing collagenase I unit/ml at pH 7.8 at 37°C with a continuous stirring. The amount of drug released into the solution was measured using a BCA protein assay kit (Pierce, Rockford, IL). The absorbance was measured at 562 nm, and the amount of chamomile extract was measured from a standard curve prepared from different concentrations of chamomile extract. An average of four replicate tests was also considered.
Cytotoxicity assays
To evaluate the cytotoxicity of the hydrogel scaffolds, two assays were designed; cell proliferation assay and morphology study. The details of cytotoxicity assays are published previously[12] and presented briefly here. First, L929 cells (mouse fibroblast) extracted and maintained in Dulbecco's modified eagles medium (DMEM)/Ham's F1 growth medium, supplemented with 1% penicillin/streptomycin (Sigma, USA), and 10% fetal bovine serum (Seromed, Germany). Cell proliferation rate was evaluated directly on sample (1.8 cm2) using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium-bromide (MTT, Sigma, USA) assay. As controls, 24 well tissue culture plates (Orange Scientific, Belgium) were used. The cultured cells in 24 well plates were washed with PBS and incubated in 400 μl of DMEM (without phenol red) containing 0.5 mg/ml MTT for 4 h at 37°C in a 5% carbon dioxide humid atmosphere incubator. After incubation, the medium was removed, and cells were washed with PBS. To develop the color, 400 μL of dimethyl sulfoxide was added and the plate shaken gently for 10 min. The absorbance of wells was measured by a multi-well microplate reader (Shimadzu, 1601) at a wavelength of 570 nm, with a reference wavelength of 650 nm, and normalized to the control measurement. The experiments were performed three times: days 1, 4, and 7. To evaluate the morphology of fibroblast cells, cultured onto the starch/4 wt% nZ and starch/extract/4 wt% nZ, the scanning electron microscope was applied out after 1, 4, and 7 days. First, the attached fibroblast cells were fixed on scaffolds by glutaraldehyde in phosphate buffer solution 2.5% and pH 7.4 for 10 min. The specimens were dehydrated in graded alcohol (10%–100%) and dried for gold sputtering.
Study design
A rat third-degree burn wound model was used to determine the efficacy of nanozeolite/starch thermoplastic hydrogel dressing to treat full thickness burns. The Ethics Committee for animal experiments at Isfahan University of Medical Sciences approved the study, and all experiments were conducted in accordance with the international guiding principles for biomedical research involving animals (revised 1985).
Animal study
A total of 48 adult male rats weighting approximately 250 g were used in this study. Before the surgical procedures, all the animals were acclimatized to the laboratory environment for several days. During the experiment, the animals were housed in cages, maintained under controlled environmental conditions (12-h light/dark cycle and temperature approximately 23°C).
The experiment was conducted for three steps. The rats were randomly divided into four groups of 4 animals each (16 rats at each step). The animals were anesthetized with intramuscular injection of ketamine hydrochloride (75 mg/kg) and xylazine hydrochloride (10 mg/kg). Immediately after anesthesia, the dorsum of animals was disinfected with alcohol and a third-degree burn injury was created.[13] Briefly, the dorsum skin was shaved and put in contact with a round hot metal rod (2 cm diameter) for 4 s. The rod has been heated in a 100°C water bath for 5 min. After cleaning all wounds by sterile normal saline, the ulcers of the first group were treated with the same size of pure hydrogels. The second group received a bandage with the same size of hydrogel/extract/4 wt% nZ (hydrogel NZE). The third group was treated with chamomile extract, and the fourth group was considered as control without taking any medicament. To quantify the rate of wound healing, the animals were sacrificed with an overdose of the above-mentioned anesthetics on days 7, 14, and 21 after the burn injury.
Morphometric assay
To assess the contraction of the wound, digital photographs were obtained from all lesions on days 7, 14, and 21 after burn injury with a defined distance from the lesion, and then, the photographs were assessed with the Image J software (Image J [NIH] National Institutes of Health, Bethesda, Maryland, USA (V. 1.3.1)). The obtained data from wound area were calculated by the following formula:[10]
CL = area T0 − area Tdaysofeuthanize × 100/area T0
Where CL = contraction level, T0 = day of the lesion induction, and Tday of euthanizatioin = 7, 14, and 21 days after the lesion induction.
Histological assessment of wound healing
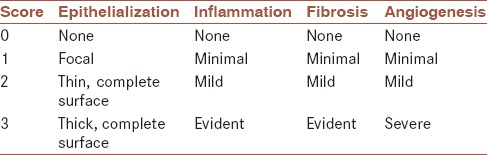
The burn areas were removed and fixed in 10% formalin. After that, they went through routine laboratory procedures to prepare paraffin block and obtain 5-μm-thick sections. The sections were stained with hematoxylin and eosin for histological analysis in light microscopy. The margins of the lesions in each section were compared with control group for histological scoring. According to the previous reports,[14,15] histologic scores were investigated based on the scoring system presented in Table 1.
Table 1.
Histopathological wound healing scale
Clinical evaluation
Five patients with chronic wounds were invited to participate in this pilot study. First, we described the starch/extract/4 wt% nZ properties with the patient and his/her care provider or legal provider. Then, we obtained written consent from the patients and the provider. Next, with collaboration with their physicians, the patients were evaluated regarding general health and specific needs. At the next step, the ulcers were examined carefully. The location, size, depth, presence of infection and necrotic tissue, and their shape were recorded. We applied the starch/extract/4 wt% nZ covering on the ulcers at 3 days intervals, and then followed the ulcers until full healing. At each visit, we took digital photos and evaluated the size changes by Images J software. Hypersensitivity reactions or any possible complication were also recorded.
Statistical analysis
Data are expressed as mean ± standard deviation. The statistical analysis of data was performed using one-way ANOVA for multiple comparisons (SPSS, Version 10.0, Chicago, Illinois, USA). A post hoc test (Tukey) was employed for determining a significance level of P < 0.05.
RESULTS
Transparency and surface morphology
The picture of our starch-based hydrogel containing 4 wt% nZ is presented in Figure 1a. As it can be seen, the nanocomposite film exhibits an appropriate transparency. Light transparencies of the scaffolds with different nZ contents were shown in Figure 1b. The results state that transparency has increased by adding the nanoparticles related to the pure starch. As shown in Figure 1b, transparency increased from 1.33 to 2.92 by increasing the nanoparticle content from 0 to 4 wt%, respectively. This demonstrates that filling the matrix with nZ affects the light transparency of hydrogels, especially untill 2 wt% nZ content. Figure 1c–g shows the surface morphology of hydrogels at 0, 1, 2, 3, and 4 wt% zeolite content. It can be observed that adding nanoparticles did not have a significant effect on the surface morphology of the samples and the figures show a homogeneous and smooth surface of all nanocomposites samples.
Figure 1.
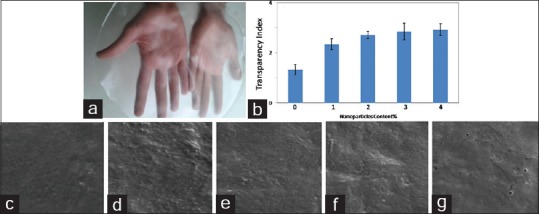
(a) Starch-based hydrogel (starch/4 wt% nZ), (b) the effect of nanozeolite particle contents on the transparency of starch-based hydrogels, and the cross-section scanning electron microscope micrographs of the hydrogels (c) starch, (d) starch/1 wt% nZ, (e) Starch/2 wt% nZ, (f) Starch/3 wt% nZ, and (g) Starch/4 wt% nZ
Swelling study
The effects of loading from 0 to 4 wt% of nZ on water uptake ability of hydrogels were evaluated. As shown in Figure 2, by increasing the nanoparticle content up to 3 wt%, the solution absorption decreased. A higher value of swelling capacity for pure starch is due to the hygroscopic nature of the starch that makes it capable for higher water uptake. This capacity was decreased by adding nZ due to forming the hydrogen bonds between matrix and nanofiller functional groups (nZ).
Figure 2.
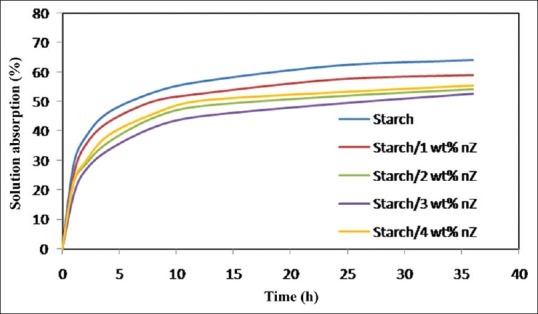
The solution uptake profile of the starch-based hydrogels at different nanozeolite contents
Drug release study
Figure 3 shows that the release profile of the samples was affected by adding nZ. The drug release profile decreased significantly by adding nZ (P < 0.05). Formulations containing 1 and 4% nZ released their full contents of drug after 8 days under the incubation condition, whereas for the pure starch formulations, full drug release took 5 days.
Figure 3.
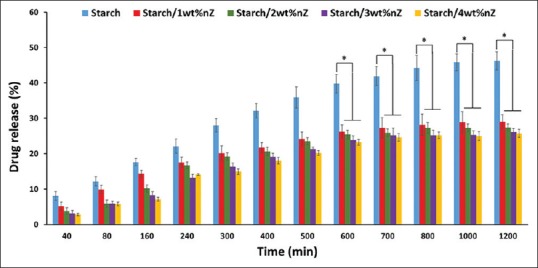
Drug-release percentage of hydrogels at different nanoparticle contents. *P < 0.05
Cytocompatibility and cell/scaffold interactions
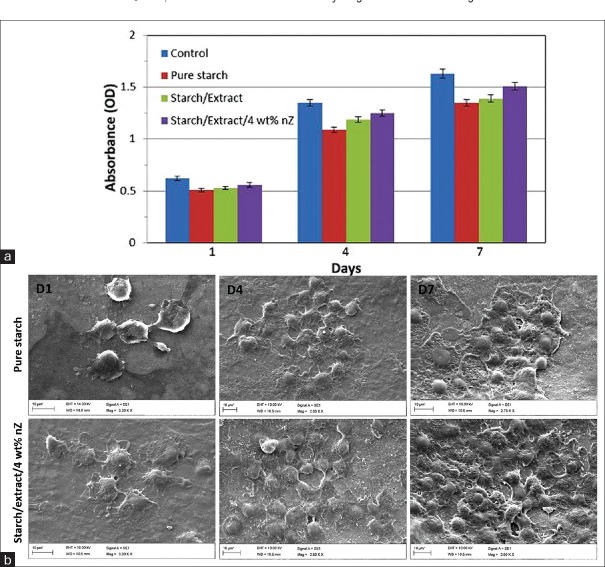
The hydrogels containing nZ were fabricated prosperous. The MTT assay was applied (according to ISO 10993-12) to analyze the cell viability and cytotoxicity of starch-based nancomposite hydrogels. As illustrated in Figure 4a, 1, 4 and 7 days after cultivation, there was not any significant difference between control and scaffold groups. Therefore, the cytotoxicity assays clearly demonstrated that there was no toxicity of the scaffold groups on fibroblast cells.
Figure 4.
(a) 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium-bromide results of L929 cells (mouse fibroblast) on tissue culture plate (as a control), pure starch, starch/extract, and starch/extract/4 wt% nZ, after 1, 4, and 7 days.(b) The morphology and density of fibroblast cells cultured on pure starch
To evaluate the cytocampatibility of hydrogels, fibroblast cells were cultured on the surface of nanocomposites. Figure 4b shows the proliferated cells after 1, 4, and 7 days on scaffolds (starch and starch/extract/4 wt% nZ). The images also show that after 4 and 7 days the cells grew on starch/extract/4 wt% nZ tended to be more mature in comparison with cells cultured on pure starch specimen.
Experimental and clinical study, animal study
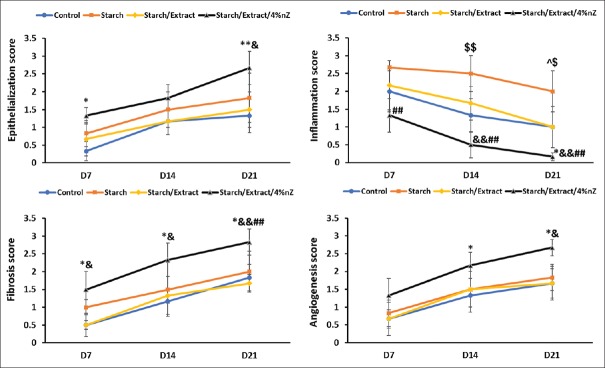
Forty-eight rats were evaluated during the experimental study. The third-degree burn wound was confirmed by histological analysis of necrosis and elimination of the structure of the epidermis, papillary dermis, reticular dermis, and hypodermis. Wound size decreased at days 7, 14, and 21 after burn injury in all groups [Figure 5]. There was a significant difference between starch/extract/4 wt% nZ and other groups in this aspect on days 7, 14, and 21 [Figure 5]. A more significant improvement in the histopathological scores of the wounds was found in the starch/extract/4 wt% nZ group compared with pure starch, extract, and control groups [Figure 6]. Our data indicated that the improvement of epithelialization, fibrosis (collagen formation), and angiogenesis scores and decrement of inflammation score in starch/extract/4 wt% nZ group [Figure 6]. These results suggest that starch/extract/4 wt% nZ dressing significantly promoted wound healing. Histological study revealed complete skin appendage regeneration especially hair follicles in starch/extract/4 wt% nZ group but incomplete epithelialization in other groups [Figure 7]. Regeneration of hair follicles was observed insufficiently in pure starch group [Figure 7]. The experiment showed starch/nZ nanocomposite, in combination with the extract, could improve wound healing in full thickness wounds better than either starch or extract treatment.
Figure 5.
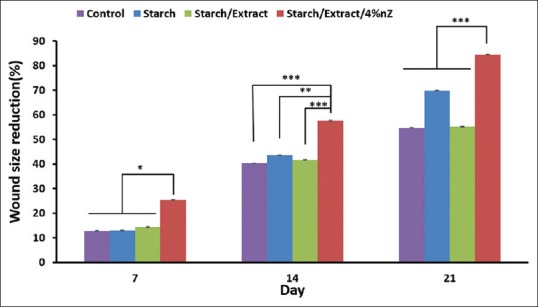
Percentage of wound size reduction of treated animals with pure starch, starch/extract, starch/extract/4 wt% nZ, and control groups in different days of the experiment. *P < 0.05 and ***P < 0.001
Figure 6.
The histopathological characteristics of the wounds of the groups. *Between starch/extract/4 wt% nZ and control groups: *P < 0.05; **P < 0.01. #Between starch/extract/4 wt% nZ and hydrogel groups: #P < 0.05; ##P < 0.01. &Between starch/extract/4 wt% nZ and extract groups: &P < 0.05; &&P < 0.01. $Between hydrogel and control groups: $P < 0.05; $$P < 0.01. ^Between hydrogel and extract groups: ^P < 0.05
Figure 7.
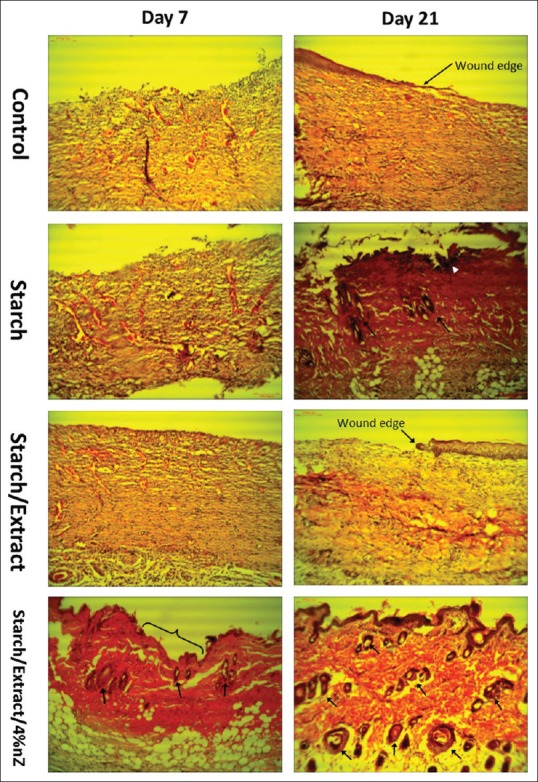
Representative images of H and E-stained histological sections at time intervals show that starch/extract/4 wt% nZ promoted wound healing. Control and extract groups showed more hyperemia without epithelialization on the days 7 and 21. In hydrogel group on day 21, there was some hair follicles (arrows), leukocyte infiltration (white arrowhead), and incomplete epithelialization. In starch/extract/4 wt% nZ group, incomplete epithelialization was observed at day 7 (curly bracket) and complete epithelialization along with a lot hair follicles at day 21 (arrows)
Clinical study
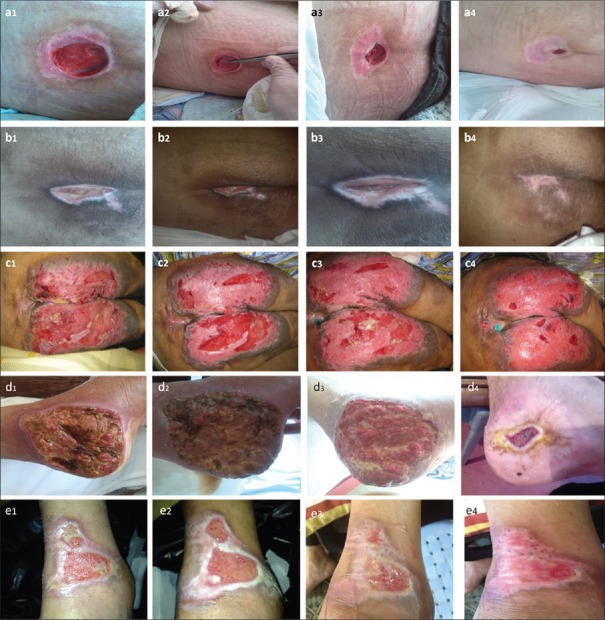
The first patient was a 23-year-old boy affected by car accident [Table 2]. Due to multiple traumas, including spinal injury and paraplegia, he developed a pressure ulcer on his back. During 30 days of nursing care, including regular wound care, there was not an improvement in the ulcer. Then starch/extract/4 wt% nZ was applied on his ulcer. His ulcer improved and finally healed during 61 days [Figure 8a].
Table 2.
Characteristics and follow up data of the patients
Figure 8.
Representative images of the refractory ulcers of five patients a-e at different time points. The images demonstrate wound surface color, and the wound surface area decreased during treatment process. The magnitude of each image may be different. a1-e1 indicate 1st day, a2, d2: day 20, b2, c2, e2: day 5, a3, d3: day 40, b3, c3, e3: day 10, a4: day 61, b4: day16, c4: day 25, d4:day 52, e1: day 15
The second patient was a 75-year-old man that because of an ischemic stroke developed a bed sore on his back [Table 1]. There was no improvement during 30 days of regular care. During 16 days of starch/extract/4 wt% nZ application his ulcer healed completely [Figure 8b].
The third patient, a 53-year-old paralyzed wheelchair dependent man, was affected by a large pressure ulcer on his buttock and thighs [Table 2]. During 10 days of care, the ulcer was enlarging fast, so we suggested him the starch/extract/4 wt% nZ scaffold. As shown in the Figure 8c, his ulcer healed during 25 days of starch/extract/4 wt% nZ application.
The fourth patient, a 73-year-old man, had syncope and affected by a burn trauma and ulcer on the medial side of the left heel up to the leg [Table 2]. After 22 days of nonhealing, we started hydrogel NZE. He felt decreasing pain after starch/extract/4 wt% nZ application. His ulcer healed during 52 days [Figure 8d].
The last patient, a 48-year-old man with a burn ulcer on the back of left foot [Table 2] for 14 days of nonhealing, was applied by starch/extract/4 wt% nZ and his ulcer improved and healed during 15 days [Figure 8e].
None of the above-mentioned patients showed hypersensitivity reactions, infection, new pain wound expansion or deepening, or any other complication. The mean duration of ulcer healing was 31 days.
DISCUSSION
In the present study, starch-based nanocomposite hydrogel scaffolds reinforced by Zeolite nanoparticles (nZ) and loaded by a herbal drug (chamomile extract) were prepared for wound dressing to accelerate the healing process.
Light transparency is an important advantage of hydrogels for wound dressing. Transparency lets wound healing to be observed without removing the hydrogel. Our results state that transparency has increased by adding the nanoparticles related to the pure starch. It may arise from the light scattering of the composite films due to the heterogeneous dispersion of nanoparticles within the starch.[16] However, further addition of nanoparticles had no significant effect on the light transparency of hydrogels. It could possibly be due to narrower diameter dispersity of nanoparticles and also a strong interaction between Zeolite A and starch.[11] It can be observed that adding nanoparticles has not had a significant effect on the surface morphology of the samples and the figures show a homogeneous and smooth surface of all nanocomposites samples. This may be due to the remarkable film forming properties of the starch thermoplastic, uniform nanoparticles dispersion, and good nanoparticle covering by matrix.[12,17]
Fluid uptake ability is one of the most compelling properties of wound dressing application. Starch is a super hydrophilic biopolymer. Using an inorganic nanoparticle is an effective way to control the water uptake ability of starch-based products as well as its mechanical properties and stability. Our results indicated that increasing the nanoparticle content up to 3 wt% increases significantly the solution absorption. Higher value of swelling capacity for pure starch is due to the hygroscopic nature of the starch which makes it capable for higher water uptake. This is decreased by adding nZ due to forming the hydrogen bonds between matrix and nanofiller functional groups (nZ). The presence of strong hydrogen bonds can be a powerful satisfactorily reason for stabilizing the starch matrix when it is immersed in an aqueous solution.[17,18] However, the increased swelling capacity of hydrogels by adding nZ to 4 wt% may arise from enhancing the capillary action in the hydrogel structure due to the agglomeration of nZ.[19]
Our data revealed that the release profile of the samples is affected by adding nZ. High value of drug release property of pure starch is due to its pore structure and hygroscopic nature which makes the hydrogel capable to exchange the drug with the aqueous solution and result in high drug releasing property. Adding nZ, owing to the form of strong intermolecular bonds, results in compact hydrogels with lower permeability than pure starch. The other compelling factor to reduce the drug release property may be the effect of nanoparticles to decrease the empty spaces of hydrogel structure. This is an effective parameter for the permeability behavior of polymeric films.[20,21]
To evaluate the cytocompatibility and cytotoxicity of hydrogels, fibroblast cells were cultured on the surface of nanocomposites. It may be caused by increasing the roughness that lead to enhance the hydrogel surface area. The hydrogel surface topography has a great impact on cell attachment and proliferation. Nanoparticles will increase the exposed surface to cell attachment and influence cell spreading and proliferation, satisfactorily.[22] The antibacterial and antioxidant activities of nZ and chamomile extract[20,23] could play a role for providing a suitable condition for attachment and proliferation of the cells on hydrogel scaffold surface.
In addition, the hydrogels provide a larger specific surface area for cell attachment and proliferation.[24] In spite of the fact that chamomile extract has antiproliferative and apoptotic effects on cancer cells, but it was approved this extract has not had any effect on normal cells.[25]
Histological evaluation indicated that the improvement of epithelialization, fibrosis (collagen formation), and angiogenesis scores and decrement of inflammation score in starch/extract/4 wt% nZ group. The decrement of inflammation score in starch/extract/4 wt% nZ group which can be due to anti-inflammatory effects of chamomile.[15,26] Chamomile exerts its anti-inflammatory effects through inhibition of lipopolysaccharide-induced prostaglandin E2 release and attenuation of cyclooxygenase (COX-2) enzyme activity without affecting the constitutive form, COX-1.[27]
Angiogenesis is a fundamental step in the healing process. It starts when macrophages are activated with tissue injury. The released proteases (plasmin and collagenase) from activated macrophages digest the blood vessel's basal membrane. Hence, the endothelial cells are exposed to angiogenic cytokines and induced to create new vessels that enters the healing tissue to supply of oxygen and nutrients.[28] Previous studies have shown the ability of starched based scaffolds to support the proliferation, adhesion, and maintenance of the phenotypic expression of endothelial cells. However, according to the previous study, luteolin (a phenolic compound of chamomile) and apigenin (a common dietary flavonoid in chamomile) had the anti-angiogenic potential because of its ability to inhibit the expression of vascular endothelial growth factor.[29,30]
According to Longo et al. study[31] chamomile stimulated re-epithelialization and collagen formation of oral wounds in rats. Some flavonoids in chamomile such as quercetin and apigenin increase collagen through inhibition of metalloproteinase activity by inhibiting matrix-metalloproteinase-1 (MMP-1) and down-regulating MMP-1 expression through an inhibition of the activator protein-1.[31,32]
The animal and clinical steps of the present study indicated that the starch/extract/4 wt% nZ dressing promotes the production of granulation tissue and epithelialization of the ulcers. As the clinical part was a small nonrandomized observational study, it can just give us some primary information about the safety and clinical effectiveness of the treatment with the starch/extract/4 wt% nZ dressing. The results are consistent with some other studies in which the local application of hydrogel dressings promotes the healing process of chronic wounds. Although we are not able to compare our findings with other studies about hydrogel, it seems that adding zeolite and chamomile to the hydrogel increased its effectiveness without endangering the patients. Hydrogels that are based on starch polymers provide moisture to the wound and encourage debridement. Hydrogels also absorb exudates and maintain necessary factors for tissue degradation and repair such as lysosomes and growth factors close to the wound.[9,10]
CONCLUSION
This research showed that starch-based nanocomposite/chamomile extract (hydrogel NZE) promotes rapid and complete skin regeneration; therefore, it has great potential to serve as a unique dressing for better treatment of selected chronic ulcers.
Financial support and sponsorship
This study was funded by a grant from Iran National Science Foundation (Grant no. 93007652).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank all the members of the laboratorys for helpful and insightful discussions. We are indebted to Mrs Amozegar for Histotechnical support.
REFERENCES
- 1.Mohamad N, Mohd Amin MC, Pandey M, Ahmad N, Rajab NF. Bacterial cellulose/acrylic acid hydrogel synthesized via electron beam irradiation: Accelerated burn wound healing in an animal model. Carbohydr Polym. 2014;114:312–20. doi: 10.1016/j.carbpol.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Moraes PR, Saska S, Barud H, Lima LR, Martins VC, Plepis AM, et al. Bacterial cellulose/collagen hydrogel for wound healing. Mat Res. 2016;19:106–16. [Google Scholar]
- 3.Mendonça RJ, Coutinho-Netto J. Cellular aspects of wound healing. An Bras Dermatol. 2009;84:257–62. doi: 10.1590/s0365-05962009000300007. [DOI] [PubMed] [Google Scholar]
- 4.Du L, Tong L, Jin Y, Jia J, Liu Y, Su C, et al. A multifunctional in situ-forming hydrogel for wound healing. Wound Repair Regen. 2012;20:904–10. doi: 10.1111/j.1524-475X.2012.00848.x. [DOI] [PubMed] [Google Scholar]
- 5.Kamoun EA, Chen X, Eldin MS, Kenawy ER. Crosslinked poly (vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arabian J Chem. 2015;8:1–14. [Google Scholar]
- 6.Naves L, Almeida L. Wound healing dressing and some composites such as zeolite, TiO2, chitosan and PLGA: A review. World Academy of Science, Engineering and Technology. Int J Med Health Biomed Bioeng Pharm Eng. 2015;9:242–6. [Google Scholar]
- 7.Jarrahi M. An experimental study of the effects of matricaria chamomilla extract on cutaneous burn wound healing in albino rats. Nat Prod Res. 2008;22:422–7. doi: 10.1080/14786410701591713. [DOI] [PubMed] [Google Scholar]
- 8.Nayak BS, Raju SS, Rao AV. Wound healing activity of matricaria recutita L. extract. J Wound Care. 2007;16:298–302. doi: 10.12968/jowc.2007.16.7.27061. [DOI] [PubMed] [Google Scholar]
- 9.Kim HG, Kim JH. Preparation and properties of antibacterial poly (vinyl alcohol) nanofibers by nanoparticles. Fiber Polym. 2011;12:602–9. [Google Scholar]
- 10.Tang X, Alavi S, Herald TJ. Barrier and mechanical properties of starch-clay nanocomposite films. Cereal Chem. 2008;85:433–9. [Google Scholar]
- 11.Nasri-Nasrabadi B, Behzad T, Bagheri R. Preparation and characterization of cellulose nanofiber reinforced thermoplastic starch composites. Fiber Polym. 2014;15:347–54. [Google Scholar]
- 12.Nasri-Nasrabadi B, Mehrasa M, Rafienia M, Bonakdar S, Behzad T, Gavanji S, et al. Porous starch/cellulose nanofibers composite prepared by salt leaching technique for tissue engineering. Carbohydr Polym. 2014;108:232–8. doi: 10.1016/j.carbpol.2014.02.075. [DOI] [PubMed] [Google Scholar]
- 13.Sun G, Zhang X, Shen YI, Sebastian R, Dickinson LE, Fox-Talbot K, et al. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc Natl Acad Sci U S A. 2011;108:20976–81. doi: 10.1073/pnas.1115973108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta V, Mittal P, Bansal P, Khokra SL, Kaushik D. Pharmacological potential of Matricaria recutita – A review. Int J Pharm Sci Drug Res. 2010;2:12–6. [Google Scholar]
- 15.Srivastava JK, Pandey M, Gupta S. Chamomile, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Life Sci. 2009;85:663–9. doi: 10.1016/j.lfs.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barka N, Qourzal S, Assabbane A, Nounah A, Ait-Ichou Y. Removal of reactive yellow 84 from aqueous solutions by adsorption onto hydroxyapatite. J Saudi Chem Soc. 2011;15:263–7. [Google Scholar]
- 17.Pereira R, Mendes A, Bártolo P. Novel alginate/aloe vera hydrogel blends as wound dressings for the treatment of several types of wounds. Chem Eng Trans. 2013;32:1009–14. [Google Scholar]
- 18.Saha N, Saarai A, Roy N, Kitano T, Saha P. Polymeric biomaterial based hydrogels for biomedical applications. J Biomater Nanobiotechnol. 2011;2:85. [Google Scholar]
- 19.Kaushik A, Singh M, Verma G. Green nanocomposites based on thermoplastic starch and steam exploded cellulose nanofibrils from wheat straw. Carbohydr Polym. 2010;82:337–45. [Google Scholar]
- 20.Kou JH, Fleisher D, Amidon GL. Modeling drug release from dynamically swelling poly (hydroxyethyl methacrylate-co-methacrylic acid) hydrogels. J Controll Release. 1990;12:241–50. [Google Scholar]
- 21.Loo SC, Tan ZY, Chow YJ, Lin SL. Drug release from irradiated PLGA and PLLA multi-layered films. J Pharm Sci. 2010;99:3060–71. doi: 10.1002/jps.22079. [DOI] [PubMed] [Google Scholar]
- 22.Wei J, Igarashi T, Okumori N, Igarashi T, Maetani T, Liu B, et al. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed Mater. 2009;4:045002. doi: 10.1088/1748-6041/4/4/045002. [DOI] [PubMed] [Google Scholar]
- 23.Abdoul-Latif FM, Mohamed N, Edou P, Ali AA, Djama SO, Obame L-C, et al. Antimicrobial and antioxidant activities of essential oil and methanol extract of Matricaria chamomilla L. from Djibouti. J Med Plants Res. 2011;5:1512–7. [Google Scholar]
- 24.Qu J, Wang L, Hu Y, Wang L, You R, Li M. Preparation of silk fibroin microspheres and its cytocompatibility. J Biomater Nanobiotechnol. 2013;4:84–90. [Google Scholar]
- 25.Srivastava JK, Gupta S. Antiproliferative and apoptotic effects of chamomile extract in various human cancer cells. J Agric Food Chem. 2007;55:9470–8. doi: 10.1021/jf071953k. [DOI] [PubMed] [Google Scholar]
- 26.Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–11. [PubMed] [Google Scholar]
- 28.Crowther M, Brown NJ, Bishop ET, Lewis CE. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol. 2001;70:478–90. [PubMed] [Google Scholar]
- 29.Santos MI, Tuzlakoglu K, Fuchs S, Gomes ME, Peters K, Unger RE, et al. Endothelial cell colonization and angiogenic potential of combined nano- and micro-fibrous scaffolds for bone tissue engineering. Biomaterials. 2008;29:4306–13. doi: 10.1016/j.biomaterials.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 30.Silva GA, Coutinho OP, Ducheyne P, Shapiro IM, Reis RL. Starch-based microparticles as vehicles for the delivery of active platelet-derived growth factor. Tissue Eng. 2007;13:1259–68. doi: 10.1089/ten.2006.0194. [DOI] [PubMed] [Google Scholar]
- 31.Longo RE. Effects of Chamomilla recutita (L.) on oral wound healing in rats. Cir Bucal. 2011;16:e716–21. [PubMed] [Google Scholar]
- 32.Romano B, Pagano E, Montanaro V, Fortunato AL, Milic N, Borrelli F, et al. Novel insights into the pharmacology of flavonoids. Phytother Res. 2013;27:1588–96. doi: 10.1002/ptr.5023. [DOI] [PubMed] [Google Scholar]