Fig. 5.
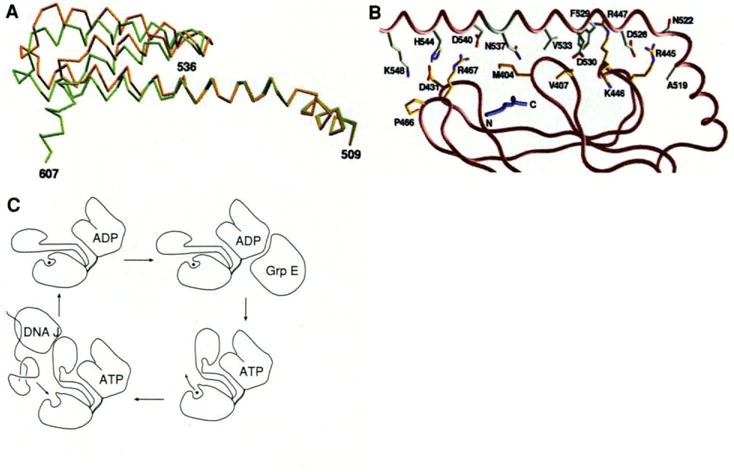
(A) Superposition of the Cα trace of the helices in two different crystal forms, based on the Cα positions in the β-sandwich subdomain (396–501). The type 2 (P21212) structure is shown in brown and the type 1 (I222) is shown in green. (B) Residues at the interface between the β-sandwich subdomain and helix αB. The orientation of the structure is similar to that in Fig, 2B, The peptide is colored blue. The carbon atoms of the residues from αB are colored white and the residues from the β domain are colored yellow. Nitrogen and oxygen atoms are colored blue and red, respectively. (C) Schematic representation of the mechanistic function of DnaK. [Parts (A) and (B) were produced by GRASP (55)]
