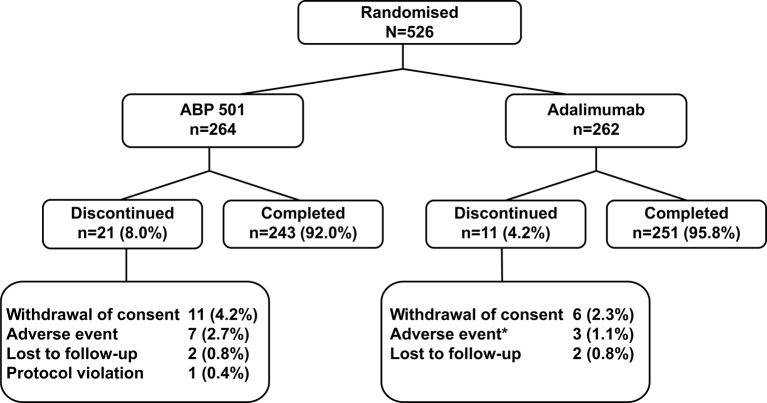
Figure 1.
Patient disposition. *n=1 patient took prohibited concomitant medication due to an adverse event and was discontinued from the study. First patient was screened on 15 October 2013 and enrolled on 24 October 2013. Patients screened, n=747; per-protocol analysis set, n=463 (ABP 501, n=230; adalimumab, n=233).