Figure 1.
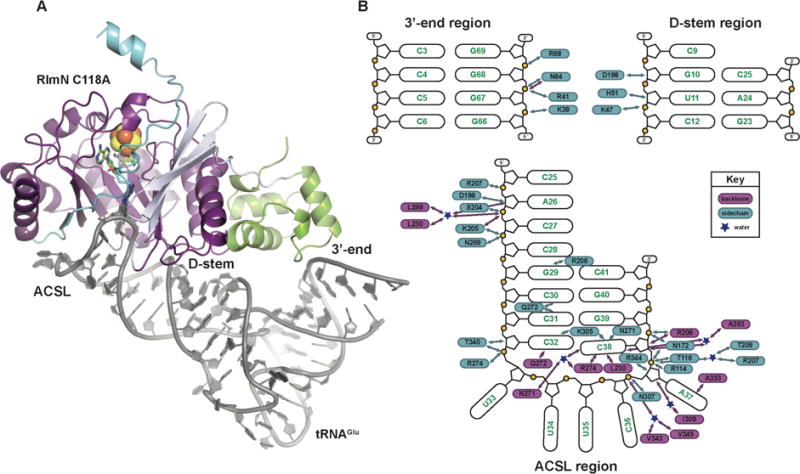
The x-ray crystal structure of the RlmN C118A-tRNAGlu in vitro cross-link. (A) The C118A RlmN protein is shown as a ribbon diagram, colored by domain, and the tRNAGlu is illustrated as a cartoon in grey. The mC355-A37 cross-link and SAM cleavage products, 5′dA and methionine, are shown in stick format and colored by atom type. The [4Fe-4S]2+ cluster is shown as a space-filling model. (B) A schematic diagram of the interactions between RlmN and the tRNA substrate. Protein residues shown in blue interact via the side chain and those shown in purple interact through the peptide backbone. Blue stars represent water molecules. The RNA is divided into three parts corresponding to the three regions of interaction observed in the complex.
