Figure 2.
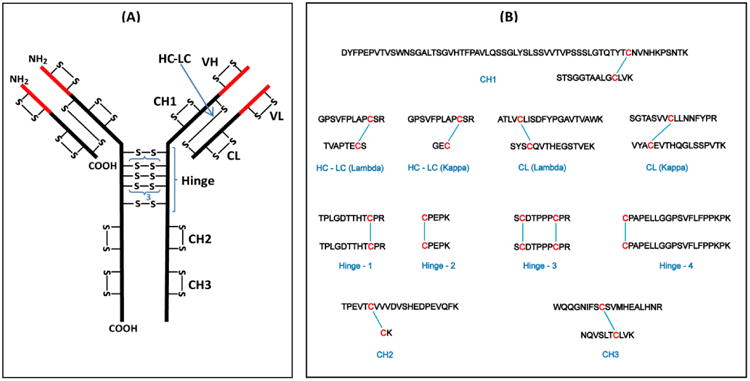
(A) Structure of a typical human IgG3 antibody showing the disulfide bond pattern. There are a total of 50 Cys residues and 25 disulfide bonds (–S–S–). The red parts are the variable (V) regions and the black parts are the constant (C) regions. H and L indicate the heavy and light chains, respectively; VL and CL are domains of the light chain; VH, CH1, CH2, and CH3 are domains of the heavy chain. The hinge region has a 15-residue segment that is repeated three times. (B) Expected tryptic dipeptides from human IgG3 constant region.
