Abstract
Introduction
The objective of this study was to determine the incidence of herpes simplex encephalitis (HSE), known as the most common, potentially mortal, and treatable cause of sporadic encephalitis, in a sample Turkish population.
Methods
The demographic, clinical, laboratory, imaging, electrophysiology, and polymerase chain reaction (PCR) DNA results of patients examined with a pre-diagnosis of encephalitis were retrospectively examined.
Results
A total of 68 patients were included in the study. The most common presenting symptom was altered behavior (67.6%), while temporal T2 hyperintensity was determined in the magnetic resonance imaging (MRI) of 27.9% of the patients and electroencephalography (EEG) abnormalities were determined in 66.2% of the patients. Lymphocytic pleocytosis was determined in the cerebrospinal fluid (CSF) in 35 patients. Fifty-seven patients had been diagnosed with viral encephalitis, 3 with bacterial meningitis, 3 with tuberculous meningitis, 2 with sporadic Creutzfeld–Jakob disease, 2 with acute disseminating encephalomyelitis, and 1 with Brucella encephalitis. Seven (10.2%) cases of viral encephalitis were found to be positive for herpes simplex virus (HSV) DNA by PCR.
Conclusion
Viral encephalitis is the most common cause of infectious encephalitis; however, other atypical causes should also be noted. Negative PCR results for HSV DNA should not exclude the need for antiviral therapy in patients with a strong pre-diagnosis of HSE because diagnostic modalities, including PCR, may fail in acute settings and HSE remains the sole treatable cause of infectious encephalitis.
Keywords: Encephalitis, herpes simplex virus, Turkish, polymerase chain reaction, encephalitis mimickers
INTRODUCTION
Encephalitis is the inflammation of the brain parenchyma, often caused by viral agents, including herpes simplex, varicella zoster, arboviridae, and rabies, followed by bacterial or parasitic agents and autoimmune or paraneoplastic mechanisms (1,2). The true incidence of the disease is mainly unknown because the diagnosis of encephalitis mainly depends on clinical suspicion in any patient with an altered mental status or consciousness, and there is no specific laboratory test or defined diagnostic criteria (1,3). The infectious, autoimmune, or degenerative causes of encephalitis are often intermingled, and despite the advanced molecular microbiology methods, the specific etiology remains unknown in most cases.
Herpes simplex type 1 (family Herpesviridae, genus simplex virus, species human herpesvirus 1) is the most common, potentially fatal cause of sporadic encephalitis in adults and is the only viral cause that has a specific treatment (2,3,4). Therefore, herpes simplex encephalitis (HSE) should be considered in any patient with suspected encephalitis, and ampirical antiviral acyclovir therapy should be initiated. Although a diagnosis of herpes encephalitis may be confirmed by polymerase chain reaction (PCR), a comprehensive evaluation, including clinical findings, imaging, electroencephalography, and cerebrospinal fluid (CSF) results, should be performed to reach a possible diagnosis of PCR-negative viral encephalitis or another alternative diagnosis in cases with HSE-resembling encephalitis.
The objective of this study was to determine the incidence of HSE confirmed by PCR assay in a sample Turkish population. We also aimed to determine the presenting symptoms, neurological examination, imaging, electrophysiology, and lumbar puncture findings and their contribution to the diagnosis in patients presenting with a clinical syndrome of encephalitis.
METHODS
This was a retrospective study where subjects were identified via an electronic search of our hospital database for patients with a clinical syndrome of encephalitis. The inclusion criteria were having a pre-diagnosis of encephalitis defined by the ICD-10 codes of A81–86, G04, G05, or B00; and presenting after June 2010, which is the date when the herpes simplex virus (HSV) DNA PCR assay was available at our hospital, and before December 2013, as the end of patient recruitment to the study. The institutional review board of Bakırköy Training and Research Hospital for Psychiatry, Neurology, and Neurosurgery examined and approved the study setup. Study procedures were performed in conformity with the Declaration of Helsinki. All the CSF viral PCR assays were performed at a single laboratory. Subjects who were primarily chronic psychiatric patients and who had undergone CSF testing to exclude potential encephalitis in the differential diagnosis of altered behavior were excluded from this study.
Sixty-eight patients hospitalized with a clinical syndrome of encephalitis were included. The hospital records of these eligible patients were searched for demographic findings, including age and sex, presenting symptoms, clinical, lumbar puncture, imaging, and electrophysiology findings, and PCR DNA results. Patients were classified as having PCR-confirmed herpes encephalitis, PCR-negative possible viral encephalitis, or an alternative diagnosis due to other confirmed pathogens, meningitis, or degenerative diseases.
Statistical Analysis
Statistical analyses were performed using the Statistical Package for Social Sciences 17.0 (SPSS Inc.; Chicago, IL, USA). Analysis of variance (ANOVA), chi-square, and Kruskal-Wallis tests were used to analyze the parametric and non-parametric data, respectively. A p value of ≤0.05 was considered statistically significant.
RESULTS
A total of 68 patients comprising 32 males (47.1%) and 36 females (52.9%) were included. The mean age was 49.7±0.7. The most common presenting symptom was altered behavior, i.e., nonsensical speech, failure to recognize family members, absurd hand and arm movements, and apathy, observed in 46 patients (67.6%). No epileptic discharges were recorded on EEG among patients presenting with altered behavior. This was followed in frequency by headache in 6 patients (8.8%), seizures in 3 (4.4%; comprising generalized tonic-clonic in 1 patient with PC-negative encephalitis, 1 patient with bacterial meningitis, and 1 patient with complex partial seizure with NMDA encephalitis), and loss of strength on the left side in 1. Twelve patients (17.6%) presented with a combination of altered behavior and headache.
Initial neurological examination was overall normal in 10 patients, whereas isolated findings of altered consciousness was determined in 42 patients. Altered consciousness was accompanied by minor hemiparesis in 6 patients, papilledema in 3, and neck stiffness in 6, while 1 patient had myoclonus. Recent medical history revealed upper respiratory tract infection in about a week ago in 13 patients, dental infection in 1 patient, and spinal cord stimulation and tonsillectomy operations in 1 patient each. There was no history of infection or medical intervention in the remaining 52 patients. Fever, as defined by a body temperature of ≥38.0 °C, was recorded as a positive finding in 42 (61.8%) patients (comprising five patients in PCR-positive, 32 patients in PCR-negative, and 5 patients in alternative diagnoses group; p=0.056).
Cranial magnetic resonance imaging (MRI) was performed in all patients and temporal T2 hyperintensity lesions were determined in 29.4% (n=20) of the patients, while other non-temporal lesions were determined in 20.6% (n=14) of the patients. These non-temporal lesions included basal ganglia hyperintensity plus cortical ribboning determined in 2 patients who were finally diagnosed with Creutzfeld-Jakob disease (CJD), multiple contrast enhanced lesions in 1 patient diagnosed with carcinomatous meningitis, and bilateral periventricular lesions in 1 patient diagnosed with acute disseminating encephalomyelitis (ADEM). No pathological findings were determined in MRI in the remaining 34 patients (50.0%). It should be noted that the 2 patients with CJD had initially presented with altered behavior and electroencephalography (EEG) findings suggestive of encephalitis, and had no cells in lumbar puncture. However, these patients had later developed myoclonus, and protein 14-3-3 positivity was determined in their CSF analysis.
In CSF examination, elevated white blood cell count was determined in 80.1% of the patients (n=55), with a median count of 40 cell/mm3 (range: 4–4800). The pleocytosis was lymphocytic in 51.5% (n=35) of the patients, and neutrophilic in 29.4% (n=20). CSF protein count was increased (>50 mg/dL) overall in 57.4% (n=37) of the patients, with a median level of 55 mg/dL (range: 17–420). Patients in the alternative diagnosis group had significantly higher levels of CSF protein compared to both the PCR-negative and PCR-positive viral encephalitis groups (p=0.001); however, the CSF protein level was comparable between the PCR-confirmed herpes encephalitis and PCR-negative possible viral encephalitis groups (p=0.992).
Overall, 55 (80.9%) patients had been diagnosed with viral encephalitis, of whom 52 improved with intravenous acyclovir 30–45 mg/kg/day treatment for 21 days. Three patients with PCR-negative viral encephalitis were deceased and permission could not be obtained for autopsy from their families. Among the subjects with viral encephalitis, 7 (10.3%) were PCR-positive for HSV-1 DNA, all of whom had improved with antiviral therapy. The specific viral agent could not be determined in the remaining 48 (70.6%) subjects, and those were noted as PCR-negative possible viral encephalitis. The clinical, imaging, electrophysiology, and lumbar puncture (LP) results of patients with PCR-confirmed viral encephalitis are presented in Table 1. In addition, Figures 1 and 2 demonstrate the EEG and cranial MRI of 2 patients with PCR-confirmed HSE and PCR-negative encephalitis, respectively.
Table 1.
Clinical, imaging, electrophysiology, and LP results of cases with PCR-confirmed viral encephalitis
| Age | Sex | Presentation | Recent medical history | MRIa | EEGb | CSFc examination |
|---|---|---|---|---|---|---|
| 58 | M | USA | URTIf | Left temporal T2 hyperintensity |
Left centrotemporoparietal BEDg | Lymphocytic (40/mm3), protein 100 mg/dL |
| 53 | F | AB | NSh | Left mesiotemporal T2 hyperintensity |
NPi | Lymphocytic (330/mm3), protein 88 mg/dL |
| 58 | F | He, AB | NS | Bilateral temporal and frontal lobe T2 hyperintensity | Diffuse BED | Neutrophilic (127/mm3), protein 66 mg/dL |
| 75 | F | AB | URTI | NS | Diffuse BED and SWj | Neutrophilic (200/mm3), protein 79 mg/dL |
| 67 | F | AB | NS | Bilateral mesiotemporal T2 hyperintensity |
Left hemisphere dominant BED on both hemispheres | Lymphocytic (30/mm3), protein 55 mg/dL |
| 26 | F | AB | Tonsillectomy | Bilateral temporal T2 hyperintensity |
BED prominent on anterior hemispheric regions and left frontotemporal neuronal hyperexcitability | Lymphocytic (570/mm3), protein 81 mg/dL |
| 55 | M | AB | NS | Bilateral temporal T2 hyperintensity |
Left temporoparietal BED | Neutrophilic (38/mm3), protein 50 mg/dL |
MRI: magnetic resonance imaging
EEG: electroencephalography
CSF: cerebrospinal fluid
AB: altered behavior
H: headache
URTI: upper respiratory tract infection
BED: bioelectrical disorganization
NS: nothing significant
NP: not performed
SW: sharp wave.
Figure 1. a, b.
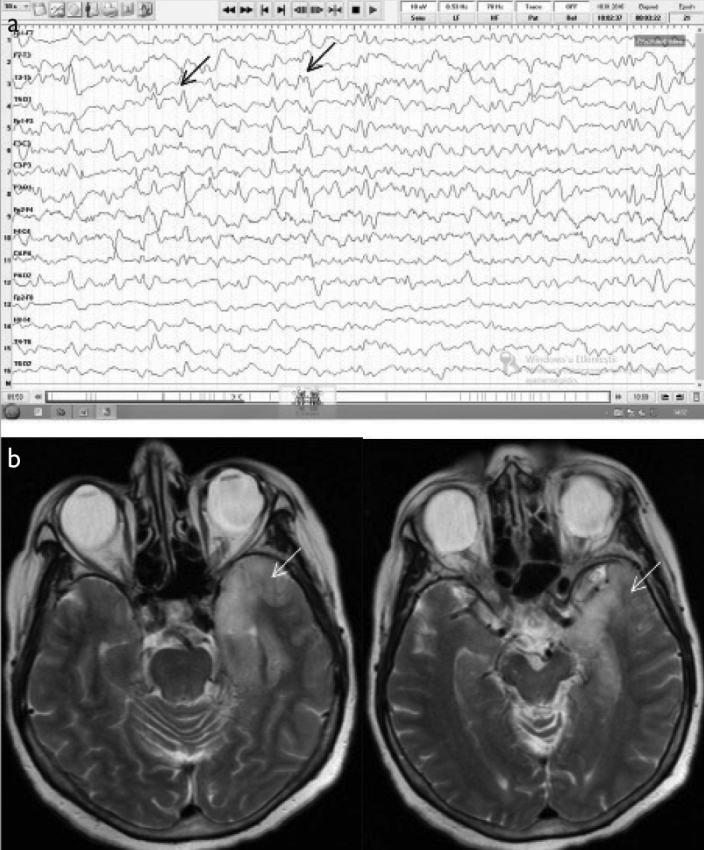
A 67-year-old female patient presenting with altered behavior was eventually diagnosed with PCR-confirmed herpes encephalitis. (a) Initial EEG (double banana montage) showed diffuse bioelectrical disorganization of 6–7 Hz frequency prominent on the left as well as repetition of sharp wave activity every 0.5 s (black arrows). (b) The cranial MRI (T2) revealed left temporal hyperintensity (white arrows).
Figure 2. a, b.
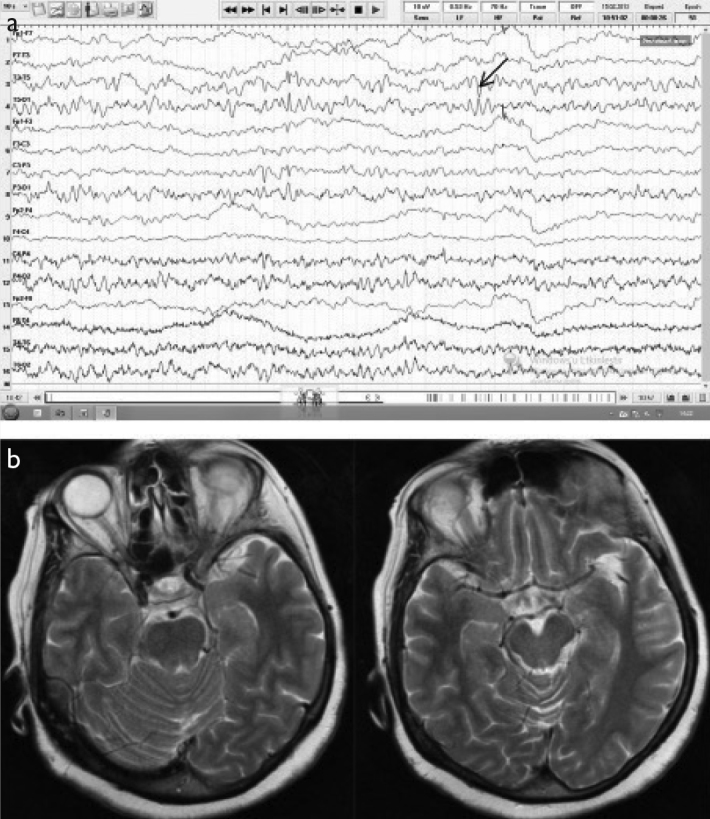
A 63-year-old female patient presenting with nonsensible speech was eventually diagnosed with PCR-negative possible viral encephalitis. (a) Initial EEG (double banana montage) showed diffuse bioelectrical disorganization on both temporal hemispheres that was more prominent on the left and temporoparietal neuronal hyperexcitability (arrow). (b) The cranial MRI (T2) was normal.
An alternative diagnosis, including bacterial meningitis, Creutzfeld-Jakob disease, non-convulsive status epilepticus, acute disseminating encephalomyelitis, Brucella encephalitis, anti-NMDA receptor encephalitis, and carcinomatous meningitis, was established in 13 (19.1%) patients. Three patients had tuberculous meningitis (Figure 3); all of whom had presented with altered behavior and were diagnosed based on CSF pleocytosis, elevated protein count, and hypoglycorrhachia, and all of whom showed clinical improvement with anti-tuberculous treatment. Two patients had non-tuberculous bacterial meningitis due to neutrophilic CSF pleocytosis (>1000 cells/mm3) and the presence of gram-positive cocci in microscopic examination of CSF. Two patients were diagnosed with sporadic Creutzfeld-Jakob disease due to the presence of periodic sharp wave complexes in EEG and myoclonus, protein 14-3-3 positivity in CSF, basal ganglia hyperintensity in T2, and restricted diffusion on diffusion weighted imaging (DWI). Non-convulsive status epilepticus was determined in 2 patients, who were initially hospitalized for suspected viral encephalitis, but turned out to be free of encephalitis due to negative viral marker results, electroencephalography findings, history of epilepsy, and response to proper anti-epileptic treatment. One patient was finally diagnosed with acute disseminating encephalomyelitis due to bilateral extensive non-contrast enhanced T2 hyperintense lesions in MRI, IgG index of 0.63 (the defined value of the laboratory was >0.6), and the response to pulse steroid therapy. One patient with NMDA antibody positivity was finally diagnosed with anti-NMDA receptor encephalitis; while another patient with characteristic MRI findings, CSF pathology findings, and concomitant lung carcinoma was diagnosed with carcinomatous meningitis with leptomeningeal metastasis, and another with PCR positivity for Brucella DNA was diagnosed with Brucella encephalitis.
Figure 3. a, b.
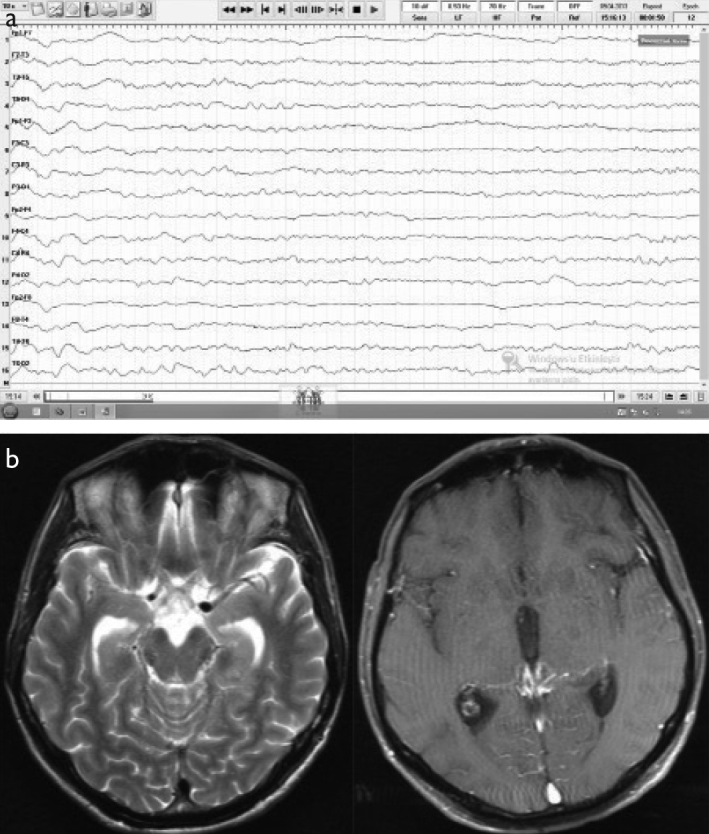
A 46-year-old male patient presenting with altered behavior was eventually diagnosed with tuberculous meningitis. (a) Initial EEG (double banana montage) showed diffuse bioelectrical disorganization of 5–6 Hz frequency on the left and 6–7 Hz frequency on the right. (b) The cranial MRI (T2 on the left and post-contrast T1 on the right) was normal.
A comparison of patients with PCR-confirmed herpes encephalitis, PCR-negative possible viral encephalitis, and an alternative diagnosis revealed that the parameters of mean age and sex were comparable between the groups (Table 2). History of a prodrome, clinical presentation, neurological examination findings, and CSF analysis were also similar between the three groups. Analysis of the MRI results by the three groups also revealed that a temporal lesion was determined in all but one PCR-confirmed HSE patient; whereas a non-temporal lesion was determined in 9 out of 13 patients with an alternative diagnosis, 5 out of 48 patients with PCR-negative possible viral encephalitis, and none of the patients with HSE. In EEG, periodic slow sharp wave paroxysmal activity was determined in 2 patients with CJD. Empirical acyclovir therapy (30 mg/kg/day applied intravenously every 8 hours in adults with normal renal function) was initiated in all patients within the first 24 hours of presentation. None of the patients presented with recurrence during the study period.
Table 2.
Comparison of patients with PCR-confirmed herpes encephalitis, PCR-negative possible viral encephalitis, or an alternative diagnosis
| Parameter | Etiology | p | ||
|---|---|---|---|---|
| PCR-confirmed Herpes encephalitis (n=7) | PCR-negative viral encephalitis (n=48) | Alternative diagnosis (n=13) | ||
| Demographic | ||||
| Female | 5 (71) | 25 (52) | 6 (46) | 0.545 |
| Age, median years (range) | (26–75) | (14–89) | (25–78) | 0.245 |
| Prodrome | ||||
| Upper respiratory tract infection | 2 (29) | 11 (23) | 1 (8) | 0.416 |
| Tonsillectomy | 1 (14) | 0 | 0 | 0.012 |
| Spinal cord stimulation operation | 0 | 1 (2) | 0 | 0.809 |
| Dental infection | 0 | 1 (2) | 0 | 0.809 |
| Clinical presentation | ||||
| Altered behavior | 6 (86) | 30 (63) | 10 (77) | 0.344 |
| Headache | 0 | 5 (10) | 1 (8) | 0.654 |
| Seizure | 0 | 1 (2) | 2 (15) | 0.098 |
| Loss of strength | 0 | 1 (2) | 0 | 0.809 |
| Altered behavior + headache | 1 (14) | 11 (23) | 0 | 0.153 |
| Neurological examination | ||||
| Altered consciousness (AC) | 5 (71) | 31 (65) | 6 (46) | 0.411 |
| Normal | 0 | 8 (17) | 2 (15) | 0.507 |
| Minor hemiparesis + AC | 1 (14) | 4 (8) | 1 (8) | 0.863 |
| Neck stiffness + AC | 1 (14) | 2 (4) | 3 (23) | 0.089 |
| Papilledema + AC | 0 | 3 (6) | 0 | 0.520 |
| Fever (≥38.0°C) | 5 (71) | 32 (66) | 5 (39) | 0.056 |
| MRIa | ||||
| Temporal T2 hyperintensity | 6 (86) | 13 (27) | 1 (8) | 0.001 |
| Non-temporal lesion | 0 | 5 (10) | 9 (69) | 0.000 |
| Normal | 1 (14) | 30 (63) | 3 (23) | 0.006 |
| EEG findings | 0.635 | |||
| Normal | 12 (25) | 1 (8) | ||
| Diffuse BEDb | 2 (29) | 13 (27) | 3 (23) | |
| Focal BED | ||||
| Left | 3 (43) | 11(23) | 3(23) | |
| Right | 4 (8) | |||
| Neuronal hyperexcitability | 1 (14) | 1 (2) | 1 (8) | |
| Generalized discharges | (-) | (-) | 1 (8) | |
| PLEDc | (-) | (-) | 2 (15) | |
| Not performed | 1 (14) | 7 (15) | 2 (15) | |
| CSF analysis | ||||
| Neutrophilic pleocytosis | 3 (43) | 12 (25) | 5 (39) | 0.456 |
| Lymphocytic pleocytosis | 4 (57) | 27 (56) | 4 (31) | 0.252 |
| No cells | 0 | 9 (19) | 4 (31) | 0.247 |
| Elevated protein count (>50 mg/dL) | 6 (86) | 25 (52) | 8 (62) | 0.230 |
MRI: magnetic resonance imaging
BED: bioelectrical disorganization
PLED: Periodic lateralized epileptiform discharges.
Summary of EEG findings
Analysis of the EEG results by the etiological study groups (Table 2) revealed that, overall, there was no statistically significant difference between the three study groups in terms of EEG findings (p=0.635). In patients with PCR-confirmed herpes encephalitis, abnormal EEG findings were reported in six out of seven patients in whom the test had been performed, comprising diffuse bioelectrical disorganization in 2 patients, left focal bioelectrical disorganization in 3, and neuronal hyperexcitability in 1. In patients with PCR-negative possible viral encephalitis, EEG was performed in 41 patients and proved normal in 12, while the remaining patients had diffuse bioelectrical disorganization, left focal bioelectrical disorganization, right focal bioelectrical disorganization, and temporal neuronal hyperexcitability in decreasing order. Finally, in patients with an alternative diagnosis, EEG was not performed in 2 patients and was interpreted as normal in 1 patient. Two patients with CJD had periodic slow sharp wave paroxysmal activity, while nonspecific findings, including diffuse bioelectrical disorganization, left focal bioelectrical disorganization, temporal neuronal hyperexcitability, and generalized discharges, were determined in the remaining 8 patients.
DISCUSSION
Herpes simplex virus is the most common cause of focal encephalitis; however, other infectious, neoplastic, cerebrovascular, or vasculitic pathologies may mimic herpes encephalitis, particularly when localized to a brain area (1). It has been demonstrated that signs and symptoms alone do not distinguish herpes from other causes of encephalitis (1,5,6). Therefore, an integrative diagnostic approach with the full utilization of neurodiagnostic techniques is a must. Imaging, laboratory studies, and clinical features often allow us to diagnose the most treatable herpes-mimicking pathologies; however, the differentiation between herpes and other causes of viral encephalitis requires more specific testing. Although brain biopsy has previously been defined as the only conclusive way to diagnose HSV (7,8), given its invasiveness and morbidity, PCR assay is the preferred diagnostic modality of viral encephalitis, with 98% sensitivity and 94% specificity (3). Furthermore, Lakeman et al. (9) suggested PCR to be superior to biopsy upon the determination of positive HSV PCR results in 3 biopsy-negative patients, possibly due to the known limitations of biopsy, including improper handling and incorrect site sampling (8).
Altered consciousness or altered behavior, followed by seizure and headache, has been reported to be the most common form of presentation in cases of acute encephalitis (2,3,10,11). Correspondingly, the most common form of presentation was altered behavior in two-thirds of the patients in our study, followed in decreasing order by headache, seizures, and loss of strength, while the most common neurological examination finding was isolated altered consciousness, observed in about two-thirds of our cases.
Laboratory findings of encephalitis are characterized with pleocytosis and elevated protein levels (1,2,3,6), with the exception of around 3 to 5 percent of patients with normal CSF analysis despite severe encephalitis, particularly initially (1,2). In our study, CSF pleocytosis was determined in 80.1% of patients, while protein levels were elevated in 57.4% of patients. These figures are comparable to those reported by Glaser et al. (5), who reported 228 out of 320 patients had CSF pleocytosis and 187 out of 320 patients had an elevated CSF protein level. However, according to our results, neither clinical presentation, nor CSF analysis differed significantly between patients with PCR-confirmed or non-confirmed encephalitis or an alternative diagnosis.
Although Steiner (3) reported that a prodromal upper respiratory tract infection is more specific to HSE, we did not determine a statistically significant difference between PCR-positive and PCR-negative patients in this respect (p=0.416, Table 2). Similar to the report of Domingues et al. (12), we determined bilateral or left temporal abnormality in MRI in 6 of the HSE patients. A comparison of MRI results between our study groups demonstrated that temporal involvement was strongly suggestive of HSE and that non-temporal involvement should entail a search for alternative etiologies. MRI was normal in only 1 patient with HSE, possibly due to the early performance of the imaging, contrary to 18 patients with PCR-negative viral encephalitis and 3 patients with an alternative diagnosis; suggesting that the absence of an MRI lesion should raise suspicion for other causes of encephalitis (Table 2). The MRI abnormalities of our HSE patients were seen in their T2 sequences and overall topographically restricted to the temporal lobe; whereas Renard et al. (13) reported a slight superiority of DWI in acute-subacute HSE. However, this discrepancy might stem from the fact that the latter study examined the MRI of HSE patients obtained within 60 days, encompassing both acute and subacute cases, whereas in our population, cranial imaging was obtained within the first 24 hours of presentation.
In addition, EEG was abnormal bilaterally or on the left in 6 patients with HSE, while CSF findings were abnormal with moderate to severe pleocytosis and moderately elevated protein levels in all HSV PCR-positive patients (Table 1). In his review, Steiner (3) also indicated the rate of CSF abnormality as >95%. However, neither EEG abnormalities nor CSF analysis differed significantly in any of our study groups (Table 2), supporting the general notion that these examination modalities remain nonspecific in the etiological search of encephalitis.
In our study, PCR-confirmed herpes simplex type-1 encephalitis was determined in 10.3% (n=7) of the cases. Furthermore, we determined unspecified viral encephalitis in 80.9% of the cases, while an alternative diagnosis, including bacterial meningitis, Creutzfeld-Jakob disease, non-convulsive status epilepticus, acute disseminating encephalomyelitis, Brucella encephalitis, anti-NMDA receptor encephalitis, and carcinomatous meningitis, was established in 19.1% of the patients. Similarly, Child et al. (10) reported the incidence of HSE as 11%, along with varicella zoster virus (VZV) in 14% and enterovirus in 1% of the cases, and an undefined etiology in 65% of the cases in their review of acute encephalitis in 37 adult patients. Kupila et al. (14) also reported HSV encephalitis in 9% of their cases, while the etiologic agent, including VZV (12%), tick-borne encephalitis virus (TBEV) (9%), and others (6%), could be determined in only one-third of the cases. On the other hand, Domingues et al. (12) reported HSE with PCR positivity in 9 out of 17 patients. We believe their contradictory 52.3% incidence, as opposed to the 10.3% in our study, ensued from the fact that only focal encephalitis cases were included in the study of Domingues.
Unfortunately, an expanded spectrum of viral DNA PCR to include VZV, enteroviridae, Epstein-Barr virus (EBV), or human herpes virus (HHV), which would probably have enlightened a significant portion of unspecified encephalitis cases, could not be performed in our hospital. Glaser et al. (5) performed one of the most extensive studies on encephalitis etiologies and examined the CSF specimens obtained from 320 patients. In this big a patient population, the incidence of HSV-1 was 3.4%; and HSV was the most common cause of infectious encephalitis, followed by enterovirus, EBV, VZV, and HHV. Glaser et al. (5) established an alternative non-infectious diagnosis in 10% of patients, and, similar to our results, the etiology was unknown in about 73% of the patients. The authors suggested that the lower percentages obtained for HSV-1 stemmed from physicians’ referral preferences, whereby physicians possibly referred the diagnostically complicated cases to the study, and thus excluded some portion of the HSE. Additionally, the inclusion of pediatric as well as adult patients might have led to their comparatively lower HSV-1 percentages.
We believe that there is a virological underdiagnosis of HSE and other viral causes of encephalitis, rather than a clinical overdiagnosis among patients hospitalized with a suspicion of encephalitis. No alternative diagnosis could be established in our cases of PCR-negative possible viral encephalitis despite extensive workup; and either one of imaging, or CSF examination, or electroencephalography findings, or a combination of these were in favor of viral encephalitis. Potential factors contributing to false negative PCR results might include the diagnostic window phase of HSV PCR in the first days of the disease, the onset of antiviral therapy prior to the performance of assay, or the presence of PCR inhibitors, including hemoglobin products, in CSF (3,15). Although some studies have reported that the sensitivity of MRI is probably not affected by the time of disease and acyclovir treatment (12), acyclovir treatment prior to CSF sampling is known to result in PCR negativity at 20 hours (16). Likewise, it has been reported that an initially negative PCR result might turn positive after the initial days of disease onset (16).
Our study had a couple of limitations. First, we retrospectively examined the computer registered data of patient records, which is blunt and thus insufficient in certain aspects, including the exact timing of CSF sampling, MRI performance with regard to the onset of symptoms, and the exact timing of the onset of acyclovir treatment. Second, serial CSF sampling, MRI imaging, or EEG recording were missing in the great majority of the PCR-negative cases. Third, PCR assays of an expanded spectrum of viral agents of encephalitis other than herpes simplex type 1 and 2 were lacking. Improvement of these limitations in future studies might allow us determine the etiology in cases of PCR-negative possible viral encephalitis, which seem to constitute the majority of cases both in our and in several literature studies, and help us to develop a better understanding of the prevalence of herpes and other causes of viral encephalitis.
In conclusion, we found the incidence of HSE confirmed by PCR to be 10.3% in our sample population. Brucella encephalitis and tuberculous meningitis stood out as important entities that should be noted in the Turkish population. Finally, our data demonstrated that other atypical causes of encephalitis and encephalitis-mimicking diseases should also be noted in clinical practice and that negative PCR results should not exclude the need for antiviral therapy in patients with a strong pre-diagnosis of HSE because diagnostic modalities, including PCR, may fail in an acute setting and HSE remains the sole treatable cause of infectious encephalitis. PCR results should be considered in light of the degree of clinical suspicion raised by presentation, EEG, lumbar puncture findings, and particularly, MRI lesions.
Footnotes
Ethics Committee Approval: Authors declared that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects”, (amended in October 2013).
Informed Consent: As the study was planned retrospectively informed consent was not needed to be taken.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - B.Ç.K., E.Ç., A.S.; Design - B.Ç.K.; Supervision - A.S., D.A., E.Ç., A.Ş.; Resources - B.Ç.K.; Materials - B.Ç.K., E.S., M.A.A.; Data Collection and/or Processing - B.Ç.K., E.S., M.A.A.; Analysis and/or Interpretation - B.Ç.K., A.S.; Literature Search - B.Ç.K.; Writing Manuscript - B.Ç.K.; Critical Review - A.S., A.Ş., D.A., E.Ç.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Whitley RJ. Viral encephalitis. N Engl J Med. 1990;323:242–250. doi: 10.1056/NEJM199007263230406. https://doi.org/10.1056/NEJM199007263230406. [DOI] [PubMed] [Google Scholar]
- 2.Silva MTT. Viral encephalitis. Arq Neuropsiquiatr. 2013;71:703–709. doi: 10.1590/0004-282X20130155. https://doi.org/10.1590/0004-282X20130155. [DOI] [PubMed] [Google Scholar]
- 3.Steiner I. Herpes simplex virus encephalitis: new infection or reactivation? Curr Opin Neurol. 2011;24:268–274. doi: 10.1097/WCO.0b013e328346be6f. https://doi.org/10.1097/WCO.0b013e328346be6f. [DOI] [PubMed] [Google Scholar]
- 4.Whitley RJ, Gnann JW. Viral encephalitis: familiar infections and emerging pathogens. Lancet. 2002;359:507–514. doi: 10.1016/S0140-6736(02)07681-X. https://doi.org/10.1016/S0140-6736(02)07681-X. [DOI] [PubMed] [Google Scholar]
- 5.Glaser CA, Gilliam S, Schnurr D, Forghani B, Honarmand S, Khetsuriani N, Fischer M, Cossen CK, Anderson LJ. In search of encephalitis etiologies: diagnostic challenges in the California Encephalitis Project, 1998–2000. Clin Infect Dis. 2003;36:731–742. doi: 10.1086/367841. https://doi.org/10.1086/367841. [DOI] [PubMed] [Google Scholar]
- 6.Kaewpoowat Q, Salazar L, Aguilera E, Wootton SH, Hasbun R. Herpes simples and varicella zoster CNS infections: clinical presentations, treatments and outcomes. Infection. 2016;44:337–345. doi: 10.1007/s15010-015-0867-6. https://doi.org/10.1007/s15010-015-0867-6. [DOI] [PubMed] [Google Scholar]
- 7.Johnson RT, Olson LC, Buescher EL. Herpes simplex virus infections of the nervous system: problems in laboratory diagnosis. Arch Neurol. 1968;18:260–264. doi: 10.1001/archneur.1968.00470330050004. https://doi.org/10.1001/archneur.1968.00470330050004. [DOI] [PubMed] [Google Scholar]
- 8.Nahmias AJ, Whitley RJ, Visintine AN, Takei Y, Alford CA., Jr Herpes simplex virus encephalitis: laboratory evaluations and their diagnostic significance. J Infect Dis. 1982;145:829–836. doi: 10.1093/infdis/145.6.829. https://doi.org/10.1093/infdis/145.6.829. [DOI] [PubMed] [Google Scholar]
- 9.Lakeman FD, Whitley RJ. Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. J Infect Dis. 1995;171:857–863. doi: 10.1093/infdis/171.4.857. https://doi.org/10.1093/infdis/171.4.857. [DOI] [PubMed] [Google Scholar]
- 10.Child N, Croxson MC, Rahnama F, Anderson NE. A retrospective review of acute encephalitis in adults in Auckland over a five-year period (2005–2009) J Clin Neurosci. 2012;19:1483–1485. doi: 10.1016/j.jocn.2012.03.010. https://doi.org/10.1016/j.jocn.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Sili U, Kaya A, Mert A. Herpes simplex virus encephalitis: clinical manifestations, diagnosis and outcome in 106 adult patients. J Clin Virol. 2014;60:112–118. doi: 10.1016/j.jcv.2014.03.010. https://doi.org/10.1016/j.jcv.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Domingues RB, Fink MCD, Tsanaclis AMC, de Castro CC, Cerri GG, Mayo MS, Lakeman FD. Diagnosis of herpes simplex encephalitis by magnetic resonance imaging and polymerase chain reaction assay of cerebrospinal fluid. J Neurol Sci. 1998;157:148–153. doi: 10.1016/s0022-510x(98)00069-0. https://doi.org/10.1016/S0022-510X(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 13.Renard D, Nerrant E, Lechiche C. DWI and FLAIR imaging in herpes simplex encephalitis: a comparative and topographical analysis. J Neurol. 2015;262:2101–2105. doi: 10.1007/s00415-015-7818-0. https://doi.org/10.1007/s00415-015-7818-0. [DOI] [PubMed] [Google Scholar]
- 14.Kupila L, Vuorinen T, Vainionpaa R, Hukkanen V, Marttila RJ, Kotilainen P. Etiology of aseptic meningitis and encephalitis in an adult population. Neurology. 2006;66:75–80. doi: 10.1212/01.wnl.0000191407.81333.00. https://doi.org/10.1212/01.wnl.0000191407.81333.00. [DOI] [PubMed] [Google Scholar]
- 15.Caliendo AM. PCR testing for the diagnosis of herpes simplex virus in patients with encephalitis or meningitis. UpToDate. 2013 [Google Scholar]
- 16.Aurelius E, Johansson B, Sköldenberg B, Staland A, Forsgren M. Rapid diagnosis of herpes simplex encephalitis by nested polymerase chain reaction assay of cerebrospinal fluid. Lancet. 1991;337:189–192. doi: 10.1016/0140-6736(91)92155-u. https://doi.org/10.1016/0140-6736(91)92155-U. [DOI] [PubMed] [Google Scholar]


