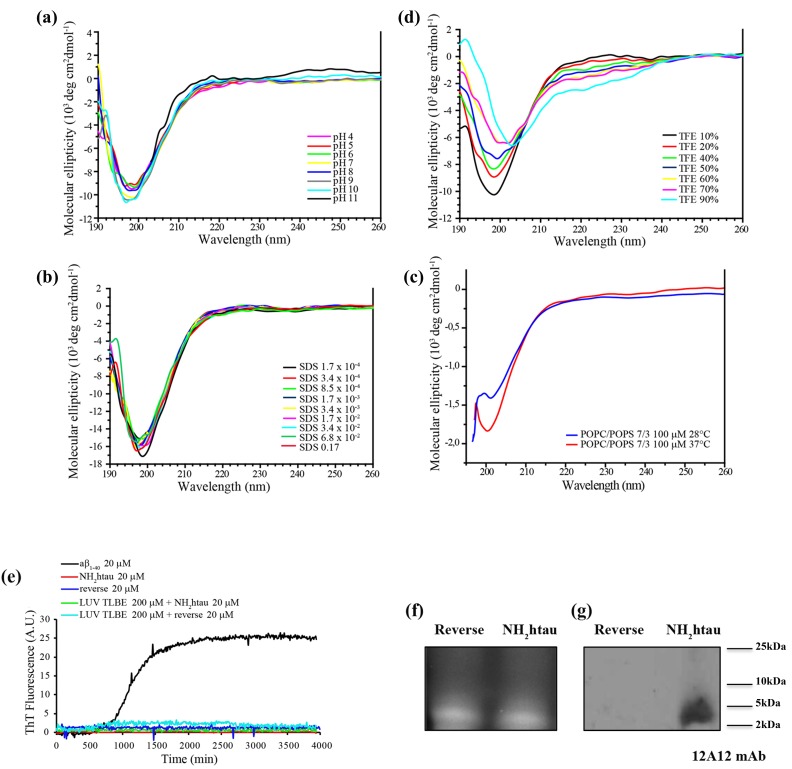
Figure 1. NH2htau 26-44 (i.e.NH2htau) shows an intrinsically disordered structure and is mainly monomeric in water environment.
a.-b.-c.-d. CD spectra of NH2htau26-44 (10µM ) at different pH(A), at different concentrations of SDS(B), in presence of large unilamellar vesicles (LUV) composed by POPC/POPS 100 µM in a 7/3 ratio at pH7.4 at increasing temperatures (c) and at different percentage of TFE(d) are shown. Notice that NH2htau shows in aqueous solution a negative peak typical for an unfolded protein with a minimum at 200nm, indicating the general absence of local major conformational changes. e. Kinetics of fiber formation was measured by ThT binding assay and ThT fluorescence of Aβ1-40 20 µM (black curve), NH2htau 20 µM in the absence (red curve) and presence of lipid LUV TLBE 200 µM (green curve), reverse control peptide (i.e reverse) 20 µM in the absence (blue curve) and presence of lipid LUV TLBE 200 µM (cyan curve) are reported. All samples were prepared in 10 mM phosphate buffer, pH 7.4, 100 mM NaCl. Time traces reported are the average of three experiments. Kinetic curves for Aβ1-40 are classically sigmoidal-shaped indicating an ongoing aggregation growth process while no noticeable differences in ThT emission intensity over time are contextually detected for NH2htau and reverse peptides both in solution and in presence of lipid membrane mimetics. f.-g. NH2htau and its reverse sequence were dissolved as described in material and methods and 150pmol of both peptides were analyzed on 15% SDS-PAGE in reducing conditions. After reversible Sypro Ruby protein staining of gel(f), Western blotting analysis was performed by probing with specific 12A12 monoclonal antibody directed against the extreme N-terminal 26-36 aa of human tau protein.Cropped representative WB is shown (g).